
Which of the following is not linear
A. \[CO_2\]
B. \[ClO_2^-\]
C. \[I_3^-\]
D. None of these
Answer
233.1k+ views
Hint: Linear geometry depicts the geometry around a central atom bonded to two other atoms positioned at a bond angle of \[180^o\].
Linear molecules like acetylene possess sp orbital hybridization for their carbon centers.
Complete step by step solution:Here in this question, we have to find out the molecule which has linear geometry.
A. \[CO_2\]
Carbon is the central atom here. It has four valence electrons.
Each oxygen atom is doubly bonded to the carbon atom. We know that in VSEPR theory, multiple bonds are considered as a single electron group.
So, there are two bond pairs on carbon.
So, it has a linear structure.
B. \[ClO_2^-\]
Chlorine is the central atom here. It has seven valence electrons.
One oxygen atom is doubly bonded and one is singly bonded to the chlorine atom. We know that in VSEPR theory, multiple bonds are considered as a single electron group.
So, there are two bond pairs and two lone pairs on the Cl atom.
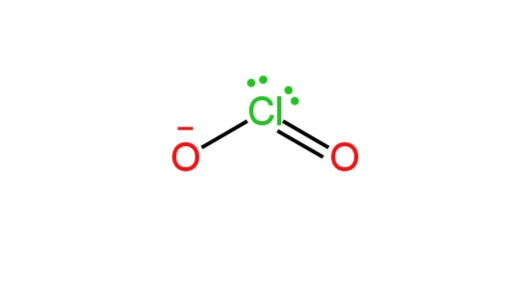
Image: Structure of \[ClO_2^-\]
So, it has a bent structure.
So, B is correct.
C. \[I_3^-\]
In this molecule, there are three Iodine atoms, one of the atoms carries a negative charge which has 3 lone pairs of electrons and 2 bond pairs.
The three lone pairs are repulsive and take equatorial positions.
So, the structure is linear.
So, C is incorrect.
So, option B is correct.
Note: To specify the total valence electrons of a molecule, aggregate all the valence electrons of the atoms present in the molecule is taken, one is subtracted for every positive charge and one is added for every negative charge.
Geometry around the central atom is determined based on the sigma bond pair and lone pair. Pi bonds are not significant.
Linear molecules like acetylene possess sp orbital hybridization for their carbon centers.
Complete step by step solution:Here in this question, we have to find out the molecule which has linear geometry.
A. \[CO_2\]
Carbon is the central atom here. It has four valence electrons.
Each oxygen atom is doubly bonded to the carbon atom. We know that in VSEPR theory, multiple bonds are considered as a single electron group.
So, there are two bond pairs on carbon.
So, it has a linear structure.
B. \[ClO_2^-\]
Chlorine is the central atom here. It has seven valence electrons.
One oxygen atom is doubly bonded and one is singly bonded to the chlorine atom. We know that in VSEPR theory, multiple bonds are considered as a single electron group.
So, there are two bond pairs and two lone pairs on the Cl atom.

Image: Structure of \[ClO_2^-\]
So, it has a bent structure.
So, B is correct.
C. \[I_3^-\]
In this molecule, there are three Iodine atoms, one of the atoms carries a negative charge which has 3 lone pairs of electrons and 2 bond pairs.
The three lone pairs are repulsive and take equatorial positions.
So, the structure is linear.
So, C is incorrect.
So, option B is correct.
Note: To specify the total valence electrons of a molecule, aggregate all the valence electrons of the atoms present in the molecule is taken, one is subtracted for every positive charge and one is added for every negative charge.
Geometry around the central atom is determined based on the sigma bond pair and lone pair. Pi bonds are not significant.
Recently Updated Pages
JEE Main Course 2026 - Important Updates and Details

JEE Main 2026 Session 1 Correction Window Started: Check Dates, Edit Link & Fees

Chemistry Question Pattern for JEE Main & Board Exams

Chemistry Question Paper PDF Download (2025, 2024) with Solutions

JEE Main Books 2026: Best JEE Main Books for Physics, Chemistry and Maths

JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




