
The volume vs. temperature graph of one mole of an ideal gas is given below:
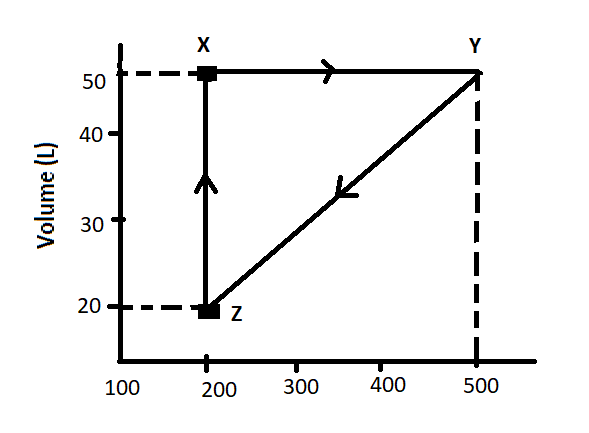
The pressure of the gas (in atm) at X, Y and Z respectively are:
[A] 0.328, 0.820, 0.820
[B] 3.28, 8.20, 3.28
[C] 0.238, 0.280, 0.280
[D] 32.8, 0.280, 82.0
Answer
233.1k+ views
Hint: To solve this question, use the ideal gas equation, PV = nRT. Find the volume and temperature for each gas using the given graph and use it to find the pressure at each of the give points X, Y and Z. Remember to use the value of the gas constant in lit-atm/mol k.
Complete step by step solution: We know that the ideal gas equation is PV = nRT where P is the pressure, V is the volume of the gas, n is the number of moles, R is the universal gas constant which has a fixed value and T is the temperature.
We can use this ideal gas equation to find out the pressure. We can get the temperature and the volume from the above graph.
Let us calculate the pressure of the gas at X-
As we can see from the graph that volume in litre for X is 50L and temperature is 200 kelvin. In the question, it is mentioned that one mole of an ideal gas is used therefore we can write that n is 1. The value of R is known to us in atm which is 0.082 lit-atm/mol K.
Therefore, putting these values in the equation, we will get-
P$\times $ 50L = 1 mol $\times $ 0.082 lit atm/mol k $\times $ 200 k
Or, $P=\dfrac{1mol\times 0.082Latm/molK\times 200K}{50L}=0.328atm$
Therefore, the pressure of one mole of an ideal gas at X is 0.0328 atm.
Now, at position Y, as we can see from the graph that volume is 50L and temperature is 500K.
Therefore, putting these values in the ideal gas equation, we will get –
$P=\dfrac{1mol\times 0.082Latm/molK\times 500K}{50L}=0.82atm$
Therefore, the pressure of one mole of an ideal gas at Y is 0.082atm.
And lastly, at position Z, we have temperature 200K and volume 20L.
Therefore, from the ideal gas law equation, the pressure will be-
$P=\dfrac{1mol\times 0.082Latm/molK\times 200K}{20L}=0.82atm$
The pressure of the ideal gas at Z is 0.82atm.
As we can see from the above discussion that pressure of one mole of the ideal gas at X, Y and Z is 0.328atm, 0.82atm and 0.82atm respectively.
Therefore, the correct answer is option [A] 0.328, 0.820, 0.820.
Note: The ideal gas law equation is an equation of state variables of a hypothetical ideal gas. It has many limitations but still used for approximation of the behaviour of a gas under certain conditions. It is a combination of Boyle’s law, Avogadro’s law, Charles’s law and Gay-Lussac’s law. However, this equation does not help us to understand whether a gas heats or cools during expansion or compression.
Complete step by step solution: We know that the ideal gas equation is PV = nRT where P is the pressure, V is the volume of the gas, n is the number of moles, R is the universal gas constant which has a fixed value and T is the temperature.
We can use this ideal gas equation to find out the pressure. We can get the temperature and the volume from the above graph.
Let us calculate the pressure of the gas at X-
As we can see from the graph that volume in litre for X is 50L and temperature is 200 kelvin. In the question, it is mentioned that one mole of an ideal gas is used therefore we can write that n is 1. The value of R is known to us in atm which is 0.082 lit-atm/mol K.
Therefore, putting these values in the equation, we will get-
P$\times $ 50L = 1 mol $\times $ 0.082 lit atm/mol k $\times $ 200 k
Or, $P=\dfrac{1mol\times 0.082Latm/molK\times 200K}{50L}=0.328atm$
Therefore, the pressure of one mole of an ideal gas at X is 0.0328 atm.
Now, at position Y, as we can see from the graph that volume is 50L and temperature is 500K.
Therefore, putting these values in the ideal gas equation, we will get –
$P=\dfrac{1mol\times 0.082Latm/molK\times 500K}{50L}=0.82atm$
Therefore, the pressure of one mole of an ideal gas at Y is 0.082atm.
And lastly, at position Z, we have temperature 200K and volume 20L.
Therefore, from the ideal gas law equation, the pressure will be-
$P=\dfrac{1mol\times 0.082Latm/molK\times 200K}{20L}=0.82atm$
The pressure of the ideal gas at Z is 0.82atm.
As we can see from the above discussion that pressure of one mole of the ideal gas at X, Y and Z is 0.328atm, 0.82atm and 0.82atm respectively.
Therefore, the correct answer is option [A] 0.328, 0.820, 0.820.
Note: The ideal gas law equation is an equation of state variables of a hypothetical ideal gas. It has many limitations but still used for approximation of the behaviour of a gas under certain conditions. It is a combination of Boyle’s law, Avogadro’s law, Charles’s law and Gay-Lussac’s law. However, this equation does not help us to understand whether a gas heats or cools during expansion or compression.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




