
The value for crystal field stabilisation energy is zero for:
(A) ${ K }_{ 2 }\left[ Mn{ F }_{ 6 } \right]$
(B) ${ K }_{ 3 }\left[ Fe(CN)_{ 6 } \right]$
(C) ${ K }_{ 3 }\left[ Fe{ F }_{ 6 } \right]$
(D) ${ K }_{ 4 }\left[ Fe(CN)_{ 6 } \right]$
Answer
232.8k+ views
Hint: Crystal field stabilization energy: Crystal field stabilization energy is the energy of electronic configuration in the ligand field - Electronic configuration in the isotropic field.
Complete answer step-by-step:
> The splitting pattern in each complex compound is octahedral. If a strong field ligand is present, then back pairing will take place as in the case of B and D, and splitting energy can never be zero, since no electron can go into ${ e }_{ g }$ orbitals.
But if a strong field is not present, then back pairing will not take place as in the case of A and C.
> The splitting pattern in ${ K }_{ 3 }\left[ Fe{ F }_{ 6 } \right]$ is;
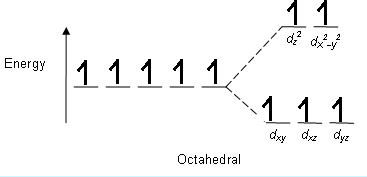
Atomic number of Fe = ${ 26 }$
Electronic configuration = $\left[ Ar \right] { 3d }^{ 6 }{ 4s }^{ 2 }$
For, ${ Fe }^{ +3 } = \left[ Ar \right] { 3d }^{ 5 } or { t }_{ 2g }^{ 3 }{ e }_{ g }^{ 2 }$
The crystal field splitting energy for octahedral complexes are given by;
CFSE = ${ -0.4\times }{ t }_{ 2g }{ +0.6\times }{ e }_{ g }$
By putting the values, we get
CFSE = ${ -0.4\times 3 }{ +0.6\times 2 }$
CFSE = 0
Now, for ${ K }_{ 2 }\left[ Mn{ F }_{ 6 } \right]$
Atomic number of Mn = ${ 25 }$
Electronic configuration = $\left[ Ar \right] { 3d }^{ 5 }{ 4s }^{ 2 }$
For, ${ Mn }^{ +3 } = \left[ Ar \right] { 3d }^{ 4 }or { t }_{ 2g }^{ 3 }{ e }_{ g }^{ 1 }$
The crystal field splitting energy for octahedral complexes are given by;
CFSE = ${ -0.4\times }{ t }_{ 2g }{ +0.6\times }{ e }_{ g }$
By putting the values, we get
CFSE = ${ -0.4\times 3 }{ +0.6\times 1 }$
CFSE = ${ -0.6 } \Delta _{ 0 }$
Hence, we can say that the value for crystal field stabilization energy is zero for ${ K }_{ 3 }\left[ Fe{ F }_{ 6 } \right]$.
The correct option is C.
Note: The possibility to make a mistake is that you may use the crystal field splitting energy formula for tetrahedral, not octahedral. But all the compounds are octahedral so do not confuse between them.
Complete answer step-by-step:
> The splitting pattern in each complex compound is octahedral. If a strong field ligand is present, then back pairing will take place as in the case of B and D, and splitting energy can never be zero, since no electron can go into ${ e }_{ g }$ orbitals.
But if a strong field is not present, then back pairing will not take place as in the case of A and C.
> The splitting pattern in ${ K }_{ 3 }\left[ Fe{ F }_{ 6 } \right]$ is;

Atomic number of Fe = ${ 26 }$
Electronic configuration = $\left[ Ar \right] { 3d }^{ 6 }{ 4s }^{ 2 }$
For, ${ Fe }^{ +3 } = \left[ Ar \right] { 3d }^{ 5 } or { t }_{ 2g }^{ 3 }{ e }_{ g }^{ 2 }$
The crystal field splitting energy for octahedral complexes are given by;
CFSE = ${ -0.4\times }{ t }_{ 2g }{ +0.6\times }{ e }_{ g }$
By putting the values, we get
CFSE = ${ -0.4\times 3 }{ +0.6\times 2 }$
CFSE = 0
Now, for ${ K }_{ 2 }\left[ Mn{ F }_{ 6 } \right]$
Atomic number of Mn = ${ 25 }$
Electronic configuration = $\left[ Ar \right] { 3d }^{ 5 }{ 4s }^{ 2 }$
For, ${ Mn }^{ +3 } = \left[ Ar \right] { 3d }^{ 4 }or { t }_{ 2g }^{ 3 }{ e }_{ g }^{ 1 }$
The crystal field splitting energy for octahedral complexes are given by;
CFSE = ${ -0.4\times }{ t }_{ 2g }{ +0.6\times }{ e }_{ g }$
By putting the values, we get
CFSE = ${ -0.4\times 3 }{ +0.6\times 1 }$
CFSE = ${ -0.6 } \Delta _{ 0 }$
Hence, we can say that the value for crystal field stabilization energy is zero for ${ K }_{ 3 }\left[ Fe{ F }_{ 6 } \right]$.
The correct option is C.
Note: The possibility to make a mistake is that you may use the crystal field splitting energy formula for tetrahedral, not octahedral. But all the compounds are octahedral so do not confuse between them.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




