
The total number of coordination sites in ethylenediaminetetraacetate () is_______.
Answer
233.1k+ views
Hint: The coordination number of an atom refers to the total number of atoms, ions, or molecules bonded to that atom in a certain molecule or crystal. The ligancy of an atom is frequently referred to as its coordination number. The ligands are the atoms, ions, or molecules bonded to the central atom (molecule/ion). The ligancy of molecules is computed differently when estimating the coordination number of a central atom in a crystal.
Complete Step by Step Solution:
\[{\left[ {C{H_2}N{{(C{H_2}C{O_2}H)}_2}} \right]_2}\] is the formula for the aminopolycarboxylic acid ethylenediaminetetraacetic acid (EDTA). two. Iron and calcium ions are typically bound with this water-soluble whitish material. As a hexadentate ("six-toothed") chelating agent, it binds these ions. Disodium EDTA, sodium calcium edetate, and tetrasodium EDTA are some of the different types of EDTA. In coordination chemistry, \[EDT{A^{4 - }}\] belongs to the aminopolycarboxylic acid family of ligands. In most cases, the two amines and four carboxylates in \[EDT{A^{4 - }}\] bind to a metal cation.
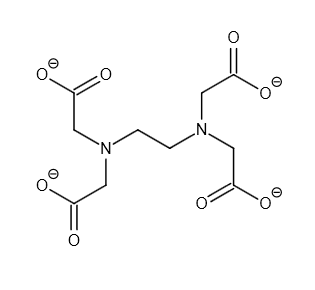
Image: Structure of \[EDT{A^{4 - }}\]
The \[EDT{A^{4 - }}\] coordination number is \[6\] . The number of ligand atoms directly linked to the core metal atom or ion via the coordinate bond is the metal's or ion's coordinate number. The number of chemical bonds produced between the ligand and the core metal atom or ion is the number of chemical bonds made between the ligand and the core metal atom or ion.
Note: Concerns about the biodegradability of aminopolycarboxylates such as EDTA have generated concern about environmental safety. As a result of these concerns, researchers are looking for alternative aminopolycarboxylates. Potential chelating agents include nitrilotriacetic acid (NTA), iminodisuccinic acid (IDS), polyaspartic acid, S,S-ethylenediamine-N,N′-disuccinic acid (EDDS), methylglycinediacetic acid (MGDA), and L-glutamic acid N,N-diacetic acid, tetrasodium salt (GLDA).
Complete Step by Step Solution:
\[{\left[ {C{H_2}N{{(C{H_2}C{O_2}H)}_2}} \right]_2}\] is the formula for the aminopolycarboxylic acid ethylenediaminetetraacetic acid (EDTA). two. Iron and calcium ions are typically bound with this water-soluble whitish material. As a hexadentate ("six-toothed") chelating agent, it binds these ions. Disodium EDTA, sodium calcium edetate, and tetrasodium EDTA are some of the different types of EDTA. In coordination chemistry, \[EDT{A^{4 - }}\] belongs to the aminopolycarboxylic acid family of ligands. In most cases, the two amines and four carboxylates in \[EDT{A^{4 - }}\] bind to a metal cation.

Image: Structure of \[EDT{A^{4 - }}\]
The \[EDT{A^{4 - }}\] coordination number is \[6\] . The number of ligand atoms directly linked to the core metal atom or ion via the coordinate bond is the metal's or ion's coordinate number. The number of chemical bonds produced between the ligand and the core metal atom or ion is the number of chemical bonds made between the ligand and the core metal atom or ion.
Note: Concerns about the biodegradability of aminopolycarboxylates such as EDTA have generated concern about environmental safety. As a result of these concerns, researchers are looking for alternative aminopolycarboxylates. Potential chelating agents include nitrilotriacetic acid (NTA), iminodisuccinic acid (IDS), polyaspartic acid, S,S-ethylenediamine-N,N′-disuccinic acid (EDDS), methylglycinediacetic acid (MGDA), and L-glutamic acid N,N-diacetic acid, tetrasodium salt (GLDA).
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




