
The structure of \[{\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\] crystal is:
(A) \[{\text{CsCl}}\]type
(B) \[{\text{NaCl}}\]type
(C) \[{\text{KCl}}\]type
(D) Anti-fluorite
Answer
242.4k+ views
Hint: Sodium oxide has a chemical formula \[{\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}{\text{.}}\] The main use of this chemical is in ceramics and in the formation of glasses. It is a base anhydride of sodium hydroxide \[{\text{(NaOH)}}{\text{.}}\] This means that when water is added to sodium oxide, sodium hydroxide is formed. The reaction is as follows –
\[N{a_2}O{\text{ }} + {\text{ }}{H_2}O{\text{ }} \to {\text{ }}2NaOH\]
Complete step by step answer:
Sodium oxide or \[{\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\] possesses the structure of anti-fluorite. Sodium ions are smaller in size than the oxide ions so they go to the voids. Moreover, the number of sodium ions present is twice that of the oxide ions so all the tetrahedral voids get filled by sodium ions. The oxide ions are larger in size so they occupy the face and corners of the lattice. Therefore, the structure formed is all the oxide ions occupy the face and corners while the sodium ions occupy the tetrahedral voids.
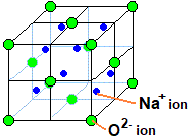
The anti-fluorite structure is derived from fluorite structure by interchanging the positive and negative positions in the lattice crystal.
Hence, the correct answer is (D) i.e., anti-fluorite.
Note: Sodium oxide is widely used in the manufacturing of ceramic and glass products. Also, sodium oxide is insoluble in water, but when added to water, it would result in the formation of sodium hydroxide.
\[N{a_2}O{\text{ }} + {\text{ }}{H_2}O{\text{ }} \to {\text{ }}2NaOH\]
Complete step by step answer:
Sodium oxide or \[{\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\] possesses the structure of anti-fluorite. Sodium ions are smaller in size than the oxide ions so they go to the voids. Moreover, the number of sodium ions present is twice that of the oxide ions so all the tetrahedral voids get filled by sodium ions. The oxide ions are larger in size so they occupy the face and corners of the lattice. Therefore, the structure formed is all the oxide ions occupy the face and corners while the sodium ions occupy the tetrahedral voids.

The anti-fluorite structure is derived from fluorite structure by interchanging the positive and negative positions in the lattice crystal.
Hence, the correct answer is (D) i.e., anti-fluorite.
Note: Sodium oxide is widely used in the manufacturing of ceramic and glass products. Also, sodium oxide is insoluble in water, but when added to water, it would result in the formation of sodium hydroxide.
Recently Updated Pages
Types of Solutions in Chemistry: Explained Simply

JEE Main Mock Test 2025-26: Principles Related To Practical

JEE Main 2025-26 Organic Compounds Containing Nitrogen Mock Test

JEE Main 2025-26 Mock Test: Organic Compounds Containing Oxygen

JEE Main 2025-26 Redox Reactions & Electro Mock Test

JEE Main Solutions Mock Test 1-2 (2025-26): Free Practice & Answers

Trending doubts
JEE Main 2026: Session 1 Results Out and Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

Ideal and Non-Ideal Solutions Explained for Class 12 Chemistry

JEE Main Participating Colleges 2026 - A Complete List of Top Colleges

Clemmensen and Wolff Kishner Reductions Explained for JEE & NEET

Degree of Dissociation: Meaning, Formula, Calculation & Uses

Understanding the Angle of Deviation in a Prism

Other Pages
CBSE Class 12 Chemistry Question Paper 2026 PDF Download (All Sets) with Answer Key

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The D And F Block Elements - 2025-26

JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 2 Electrochemistry - 2025-26

JEE Advanced 2026 - Exam Date (Released), Syllabus, Registration, Eligibility, Preparation, and More




