
The structure and hybridization of \[Si{(C{H_3})_4}\]is
(a) Bent, \[s{p^{}}\]
(b) Trigonal, \[s{p^2}\]
(c) Octahedral, \[s{p^3}d\]
(d) Tetrahedral, \[s{p^3}\]
Answer
233.1k+ views
Hint: The methane \[C{H_4}\] molecule is the best example of\[s{p^3}\]hybridization. In which one \[s\]and three\[p\]orbital are involved in hybridization and form four \[s{p^3}\]hybridized orbital of the same energy. These four orbitals have a tetrahedral arrangement around the carbon center. Other examples of \[s{p^3}\]hybridization involve \[{C_2}{H_6}\]and \[Si{(C{H_3})_4}\], etc.
Complete step by step solution:The process of hybridization involves the mixing of atomic orbitals of an atom to produce new atomic orbitals of different shapes, sizes, and energy. These new orbitals are called hybrid orbitals.
In hybridization, the half-and fully filled orbital can take part.
By employing the concept of hybridization, we can easily predict the geometry of the molecules.
Based on the mixing of orbitals, the hybridization can be classified in the following way:
(1) \[s{p^{}}\]hybridization: Involve mixing of one \[s\]and one\[p\]orbitals. It has 50% \[s\]and 50%\[p\]characters with linear geometry. E.g., the acetylene molecule.
(2) \[s{p^2}\]hybridization: Involve mixing of one \[s\]and two\[p\]orbitals. It has 33.33% \[s\]and 66.66%\[p\]character with trigonal planar geometry. E.g., \[B{H_3}\] molecule.
(3) \[s{p^3}\]hybridization: Involve mixing of one \[s\]and three\[p\]orbitals. It has 25% \[s\]and 75%\[p\]character with tetrahedral geometry. E.g., \[C{H_4}\] molecule.
(4) \[s{p^3}d\]hybridization: Involves mixing of one \[s\], three\[p\]and one \[d\] orbitals. It has trigonal bipyramidal geometry. E.g., \[PC{l_5}\] molecule.
(5) \[s{p^3}{d^2}\]hybridization: Involves mixing of one \[s\], three\[p\]and one \[d\] orbitals. It has octahedral geometry. E.g., sulfur hexafluoride.
The tetramethyl silane (\[Si{(C{H_3})_4}\]) or TMS has a very similar structure to the methane (\[C{H_4}\]) molecule. In \[Si{(C{H_3})_4}\] molecule one \[s\]and three\[p\]orbital involved in hybridization and produce four \[s{p^3}\]hybridized orbitals. These four orbitals have a tetrahedral arrangement around the silicon (\[Si\])center and form four sigma bonds with four\[C{H_3}\] groups.
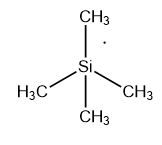
Image: structure of tetramethylsilane.
Therefore from the above explanation we can say option (d) will be the correct option:
Note: tetramethyl silane is a volatile organometallic compound.
In NMR spectroscopy tetramethyl silane is used as an internal standard to calibrate the chemical shift.
tetramethyl silane is responsible for memory loss and brain tumors in human beings.
In organic chemistry tetramethyl silane acts as a protecting group for alcohols.
Complete step by step solution:The process of hybridization involves the mixing of atomic orbitals of an atom to produce new atomic orbitals of different shapes, sizes, and energy. These new orbitals are called hybrid orbitals.
In hybridization, the half-and fully filled orbital can take part.
By employing the concept of hybridization, we can easily predict the geometry of the molecules.
Based on the mixing of orbitals, the hybridization can be classified in the following way:
(1) \[s{p^{}}\]hybridization: Involve mixing of one \[s\]and one\[p\]orbitals. It has 50% \[s\]and 50%\[p\]characters with linear geometry. E.g., the acetylene molecule.
(2) \[s{p^2}\]hybridization: Involve mixing of one \[s\]and two\[p\]orbitals. It has 33.33% \[s\]and 66.66%\[p\]character with trigonal planar geometry. E.g., \[B{H_3}\] molecule.
(3) \[s{p^3}\]hybridization: Involve mixing of one \[s\]and three\[p\]orbitals. It has 25% \[s\]and 75%\[p\]character with tetrahedral geometry. E.g., \[C{H_4}\] molecule.
(4) \[s{p^3}d\]hybridization: Involves mixing of one \[s\], three\[p\]and one \[d\] orbitals. It has trigonal bipyramidal geometry. E.g., \[PC{l_5}\] molecule.
(5) \[s{p^3}{d^2}\]hybridization: Involves mixing of one \[s\], three\[p\]and one \[d\] orbitals. It has octahedral geometry. E.g., sulfur hexafluoride.
The tetramethyl silane (\[Si{(C{H_3})_4}\]) or TMS has a very similar structure to the methane (\[C{H_4}\]) molecule. In \[Si{(C{H_3})_4}\] molecule one \[s\]and three\[p\]orbital involved in hybridization and produce four \[s{p^3}\]hybridized orbitals. These four orbitals have a tetrahedral arrangement around the silicon (\[Si\])center and form four sigma bonds with four\[C{H_3}\] groups.

Image: structure of tetramethylsilane.
Therefore from the above explanation we can say option (d) will be the correct option:
Note: tetramethyl silane is a volatile organometallic compound.
In NMR spectroscopy tetramethyl silane is used as an internal standard to calibrate the chemical shift.
tetramethyl silane is responsible for memory loss and brain tumors in human beings.
In organic chemistry tetramethyl silane acts as a protecting group for alcohols.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




