
The number of unpaired electrons \[{{\rm{O}}_{\rm{2}}}\] molecule is
A) 0
B) 1
C) 2
D) 3
Answer
233.1k+ views
Hint: The electrons of an atom that does not participate in the bonding process are called unpaired electrons. These electrons are also named lone pair electrons. Here, to find out the unpaired electrons, we have to first know the valence electrons.
Complete Step by Step Answer:
Let's first discuss what the valence electron is. They are the electrons that reside in the outermost shell of the atom. They take part in bond formation.
Let's discuss how a molecule of oxygen forms. The count of valence electrons in the Oxygen atom is six. The oxygen molecule is formed due to the formation of the covalent bond between the two atoms by sharing electrons so that they attain stability by achieving noble gas configuration, that is, valence electrons of eight.
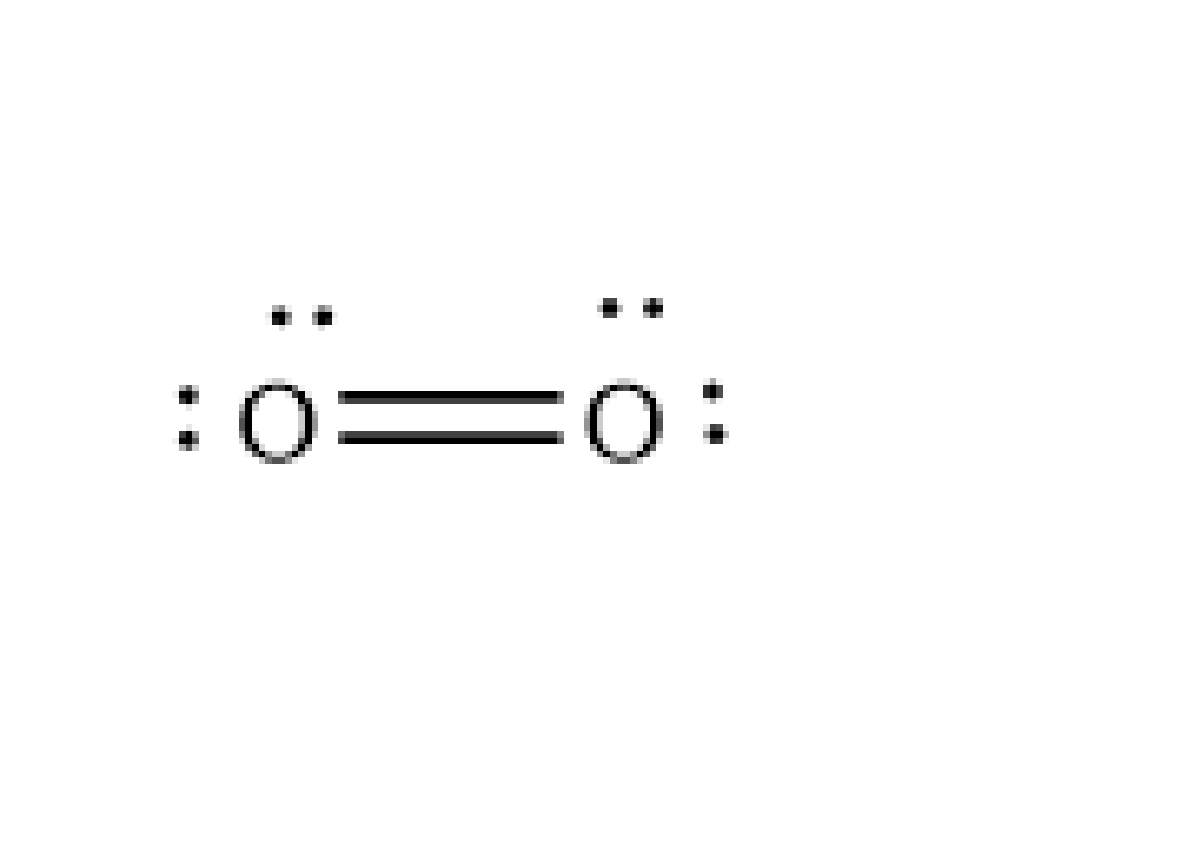
Image: Oxygen molecule
From the above diagram, it is clear that each oxygen atom shares two electrons with the other atoms to form the double covalent bond so that they achieve an octet. And each atom of oxygen has two lone pairs on it. And they are not involved in the formation of bonds. So, two lone pairs are present on the oxygen atom.
Hence, option C is right.
Note: It is to be noted that a covalent bond forms because of the sharing of electrons by the bonded atoms. If one electron is shared, the bond formed is a single bond. And the sharing of two electrons gives a double bond and the sharing of three electrons gives a triple bond.
Complete Step by Step Answer:
Let's first discuss what the valence electron is. They are the electrons that reside in the outermost shell of the atom. They take part in bond formation.
Let's discuss how a molecule of oxygen forms. The count of valence electrons in the Oxygen atom is six. The oxygen molecule is formed due to the formation of the covalent bond between the two atoms by sharing electrons so that they attain stability by achieving noble gas configuration, that is, valence electrons of eight.
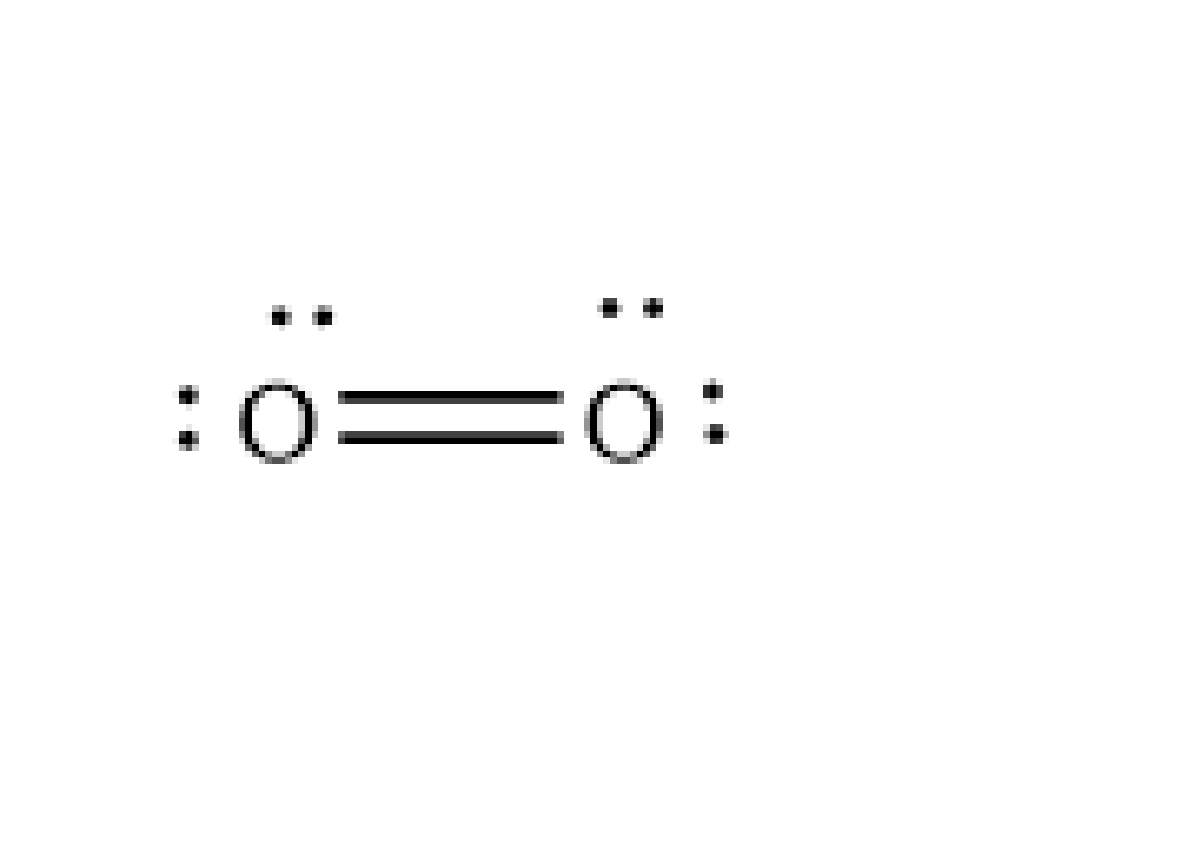
Image: Oxygen molecule
From the above diagram, it is clear that each oxygen atom shares two electrons with the other atoms to form the double covalent bond so that they achieve an octet. And each atom of oxygen has two lone pairs on it. And they are not involved in the formation of bonds. So, two lone pairs are present on the oxygen atom.
Hence, option C is right.
Note: It is to be noted that a covalent bond forms because of the sharing of electrons by the bonded atoms. If one electron is shared, the bond formed is a single bond. And the sharing of two electrons gives a double bond and the sharing of three electrons gives a triple bond.
Recently Updated Pages
Types of Solutions in Chemistry: Explained Simply

JEE General Topics in Chemistry Important Concepts and Tips

JEE Extractive Metallurgy Important Concepts and Tips for Exam Preparation

JEE Amino Acids and Peptides Important Concepts and Tips for Exam Preparation

JEE Atomic Structure and Chemical Bonding important Concepts and Tips

Electricity and Magnetism Explained: Key Concepts & Applications

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




