
The name of the compound having the structure $ClCH_2CH_2COOH$ is
A. 3-chloropropanoic acid
B. 2-chloropropanoic acid
C. 2-chloroethanoic acid
D. Chlorosuccinic acid
Answer
232.8k+ views
Hint: The given compound contains halogen chlorine and a carboxylic acid, so the compound’s name will have the suffix oic acid because carboxylic acid has higher priority than halogen group and halo that is chloro for chlorine will be used as a prefix.
Complete Step-by-step answer:
The structure of the compound is $ClCH_2CH_2COOH$ the chlorine is attached to the third carbon because the numbering starts from the carboxylic acid carbon. The name of the compound is 3-chloropropanoic acid.
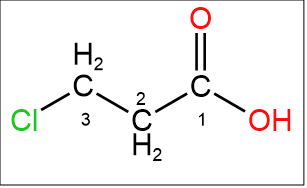
Image: 3-chloropropanoic acid
3-chloropropionic acid is a crystalline solid, its color is white. The 3-chloropropionic acid has a strong pungent smell. It has a higher density than water. 3-chloropropionic acid is used as a drug and also used as an intermediate in chemical preparations. It is soluble in water, alcohol, and chloroform. It is corrosive in nature and can burn your skin or irritate it when it comes in contact with the skin, it can also injure the eyes, and it is harmful if taken orally and can cause unconsciousness. It is used as a herbicide and does not degrade microbially, hence hazardous in nature.
The name of the given compound having a structural formula is 3-chloropropanoic acid.
Thus, Option (A) is correct
Note: It can be prepared by the hydrolysis of beta-chloropropionaldehyde or by the hydrolysis of ethylene cyanohydrin with hydrochloric acid. It produces hazardous and toxic chlorine fumes while burning which can cause irritation and can affect the respiratory tract. The inhalation of these chlorine fumes or 3-chloropropanoic acid can cause pneumonitis. 2-chloropropionic acid is an isomer of 3-chloropropanoic acid and it is also toxic in nature.
Complete Step-by-step answer:
The structure of the compound is $ClCH_2CH_2COOH$ the chlorine is attached to the third carbon because the numbering starts from the carboxylic acid carbon. The name of the compound is 3-chloropropanoic acid.

Image: 3-chloropropanoic acid
3-chloropropionic acid is a crystalline solid, its color is white. The 3-chloropropionic acid has a strong pungent smell. It has a higher density than water. 3-chloropropionic acid is used as a drug and also used as an intermediate in chemical preparations. It is soluble in water, alcohol, and chloroform. It is corrosive in nature and can burn your skin or irritate it when it comes in contact with the skin, it can also injure the eyes, and it is harmful if taken orally and can cause unconsciousness. It is used as a herbicide and does not degrade microbially, hence hazardous in nature.
The name of the given compound having a structural formula is 3-chloropropanoic acid.
Thus, Option (A) is correct
Note: It can be prepared by the hydrolysis of beta-chloropropionaldehyde or by the hydrolysis of ethylene cyanohydrin with hydrochloric acid. It produces hazardous and toxic chlorine fumes while burning which can cause irritation and can affect the respiratory tract. The inhalation of these chlorine fumes or 3-chloropropanoic acid can cause pneumonitis. 2-chloropropionic acid is an isomer of 3-chloropropanoic acid and it is also toxic in nature.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




