
The monomer unit of the given polymer is :
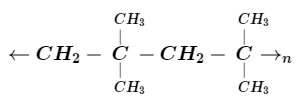
A. 
B. 
C. 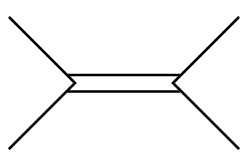
D. 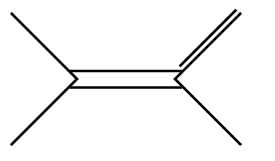
Answer
232.8k+ views
Hint : We know that a repeating unit produces the complete polymer or we can say that the polymers are formed by the repetition of a specific unit. These units are called basic units of polymer. A monomer reacts with another monomer to form a larger polymer. Polymers are composed of multiples of simpler units known as monomers.
Complete step by step solution:
We can identify monomer units in any polymer because these monomer units are the building block of the polymer. Here in the problem we are given a section of polymer and asked to identify the monomer so to solve this problem we need to pick a point and then look across and check when the next repetition point came. By doing this trick we will get a repeating unit of the polymer. On observing the given structure we come to know that the repeating unit is $ - C{H_2}C{(C{H_3})_2} - $ which is known as isobutylene. In isobutylene double bond breaks and another molecule of isobutylene attached to it hence the chain continues. So here option A is the correct answer to this problem because it is the structural representation of isobutylene$(2 - $ methylpropene) which is a colourless flammable gas.
Note : We know that polymers are very useful in our daily life. Polymer formation exactly takes place by bonding of carbon atoms one to the next into a long chain. These long chains are called the backbone of polymers. Polymers used to be very resistant to chemicals. They act as thermal and electrical insulators. They had a broad range of uses in industries also.
Complete step by step solution:
We can identify monomer units in any polymer because these monomer units are the building block of the polymer. Here in the problem we are given a section of polymer and asked to identify the monomer so to solve this problem we need to pick a point and then look across and check when the next repetition point came. By doing this trick we will get a repeating unit of the polymer. On observing the given structure we come to know that the repeating unit is $ - C{H_2}C{(C{H_3})_2} - $ which is known as isobutylene. In isobutylene double bond breaks and another molecule of isobutylene attached to it hence the chain continues. So here option A is the correct answer to this problem because it is the structural representation of isobutylene$(2 - $ methylpropene) which is a colourless flammable gas.
Note : We know that polymers are very useful in our daily life. Polymer formation exactly takes place by bonding of carbon atoms one to the next into a long chain. These long chains are called the backbone of polymers. Polymers used to be very resistant to chemicals. They act as thermal and electrical insulators. They had a broad range of uses in industries also.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




