
The correct order of increasing the basic nature of the bases ammonia, methylamine, and ethylamine in the gas phase is :
A. \[{\left( {{\rm{C}}{{\rm{H}}_{\rm{3}}}} \right)_{\rm{2}}}{\rm{NH < N}}{{\rm{H}}_{\rm{3}}}{\rm{ < C}}{{\rm{H}}_{\rm{3}}}{\rm{N}}{{\rm{H}}_{\rm{2}}}\]
B. \[{\rm{N}}{{\rm{H}}_{\rm{3}}}{\rm{ < C}}{{\rm{H}}_{\rm{3}}}{\rm{N}}{{\rm{H}}_{\rm{2}}}{\rm{ < }}{\left( {{\rm{C}}{{\rm{H}}_{\rm{3}}}} \right)_{\rm{2}}}{\rm{NH}}\]
C. \[{\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{N}}{{\rm{H}}_{\rm{2}}}{\rm{ < }}{\left( {{\rm{C}}{{\rm{H}}_{\rm{3}}}} \right)_{\rm{2}}}{\rm{NH < N}}{{\rm{H}}_{\rm{3}}}\]
D. \[{\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{N}}{{\rm{H}}_{\rm{2}}}{\rm{ < N}}{{\rm{H}}_{\rm{3}}}{\rm{ < }}{\left( {{\rm{C}}{{\rm{H}}_{\rm{3}}}} \right)_{\rm{2}}}{\rm{NH}}\]
Answer
232.8k+ views
Hint: Amines are organic compounds developed by substituting one or more hydrogen atoms with alkyl or aryl groups. The nitrogen atom in amine has a lone pair of electrons, which is why it is basic.
Complete Step by Step Solution:
Lewis's concept states that a base is a substance that can give a pair of electrons to another substance.
The nitrogen atom in amine has a lone pair of electrons. So, amines are basic.
The basicity of amines is due to their structure.
Amines provide a pair of electrons to another substance forming an ammonium cation.
The more stable this cation, the more will be its basicity.
The basicity of amines in the gaseous phase is due to the +I-effect of the alkyl groups.
Dimethyl amine
It loses a pair of electrons and forms an ammonium cation.
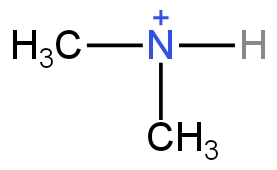
Image: Cation formed by dimethylamine.
Alkyl groups are the electron releasing group or +I group.
Here, two methyl groups are present, due to this the positive charge present on the cation gets dispersed. This increases the stability of the cation.
Methyl amine
It loses a pair of electrons and forms an ammonium cation.
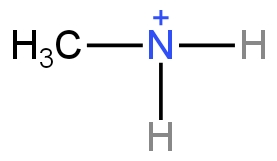
Image: Cation formed by methylamine.
Alkyl groups are the electron releasing group or +I group.
Here one methyl group is present, due to this the positive charge present on the cation gets dispersed. This increases the stability of the cation. But methyl amine is less basic than Dimethylamine.
Ammonia
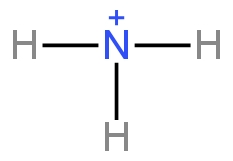
Image: Cation formed by ammonia.
Here no methyl groups are present. The positive charge on the carbon is not stabilised.
So, ammonia is the least basic out of the given options.
So, the basicity of amines should be in the order
\[{\rm{N}}{{\rm{H}}_{\rm{3}}}{\rm{ < C}}{{\rm{H}}_{\rm{3}}}{\rm{N}}{{\rm{H}}_{\rm{2}}}{\rm{ < }}{\left( {{\rm{C}}{{\rm{H}}_{\rm{3}}}} \right)_{\rm{2}}}{\rm{NH}}\]
So, option B is correct.
Note: Dimethyl amine is more basic than methyl amine and ammonia as the cation formed by Dimethyl amine is most stable. Two methyl groups present help in positive charge delocalization. There is one methyl group present in methyl amine due to which its cation is stable but not as stable as in the case of ethyl amine. Ammonia is the least stable as it has no presence of the methyl group.
Complete Step by Step Solution:
Lewis's concept states that a base is a substance that can give a pair of electrons to another substance.
The nitrogen atom in amine has a lone pair of electrons. So, amines are basic.
The basicity of amines is due to their structure.
Amines provide a pair of electrons to another substance forming an ammonium cation.
The more stable this cation, the more will be its basicity.
The basicity of amines in the gaseous phase is due to the +I-effect of the alkyl groups.
Dimethyl amine
It loses a pair of electrons and forms an ammonium cation.

Image: Cation formed by dimethylamine.
Alkyl groups are the electron releasing group or +I group.
Here, two methyl groups are present, due to this the positive charge present on the cation gets dispersed. This increases the stability of the cation.
Methyl amine
It loses a pair of electrons and forms an ammonium cation.

Image: Cation formed by methylamine.
Alkyl groups are the electron releasing group or +I group.
Here one methyl group is present, due to this the positive charge present on the cation gets dispersed. This increases the stability of the cation. But methyl amine is less basic than Dimethylamine.
Ammonia

Image: Cation formed by ammonia.
Here no methyl groups are present. The positive charge on the carbon is not stabilised.
So, ammonia is the least basic out of the given options.
So, the basicity of amines should be in the order
\[{\rm{N}}{{\rm{H}}_{\rm{3}}}{\rm{ < C}}{{\rm{H}}_{\rm{3}}}{\rm{N}}{{\rm{H}}_{\rm{2}}}{\rm{ < }}{\left( {{\rm{C}}{{\rm{H}}_{\rm{3}}}} \right)_{\rm{2}}}{\rm{NH}}\]
So, option B is correct.
Note: Dimethyl amine is more basic than methyl amine and ammonia as the cation formed by Dimethyl amine is most stable. Two methyl groups present help in positive charge delocalization. There is one methyl group present in methyl amine due to which its cation is stable but not as stable as in the case of ethyl amine. Ammonia is the least stable as it has no presence of the methyl group.
Recently Updated Pages
Types of Solutions in Chemistry: Explained Simply

JEE General Topics in Chemistry Important Concepts and Tips

JEE Extractive Metallurgy Important Concepts and Tips for Exam Preparation

JEE Amino Acids and Peptides Important Concepts and Tips for Exam Preparation

JEE Atomic Structure and Chemical Bonding important Concepts and Tips

Electricity and Magnetism Explained: Key Concepts & Applications

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




