
The chlorobenzene is generally obtained from a corresponding diazonium salt by reacting it with
A. \[C{u_2}C{l_2}\]
B. \[CuS{O_4}\]
C. \[Cu\]
D. \[Cu(N{H_3})_4^{2 + }\]
Answer
233.1k+ views
Hint: As we know that the equivalent haloarene is produced when a diazonium salt is treated with copper \[(I)\] chloride or copper \[(I)\] bromide. The Sandmeyer reaction states the name of this process. The benzene ring can then have a chloro or bromo group added to it.
Complete step-by-step answer:We must explain how the Sandmeyer reaction will be used to produce chlorobenzene from benzene diazonium chloride in order to answer the question.
As a result, aryl halides are produced from aryl diazonium salts using the Sandmeyer reaction, a chemical process. Traugott Sandmeyer, a Swiss chemist, is honored by the name of this reaction. The Sandmeyer reaction, which is frequently catalysed by copper \[(I)\] salts that is a procedure for the substituting an aromatic amino group by preparing its diazonium salt which is followed by its displacement. It is typically catalysed by copper salts. The nucleophile might be anything from cyanide to thiols to water to halide anions.
When cuprous chloride or cuprous bromide are applied to diazonium salt, respectively, chlorobenzene or bromobenzene is generated.
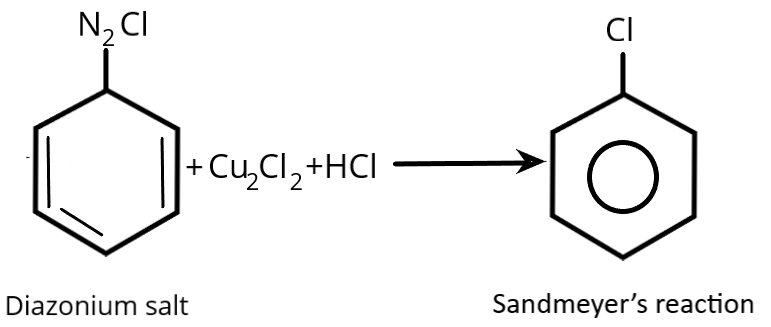
Option ‘A’ is correct
Note: It should be noted that an aromatic primary amine is processed with \[NaN{O_2}\] and dilute\[HCl\] at low temperature to produce benzene diazonium salt. The action is referred to as diazotization. The chemicals called diazonium salts are very reactive. A great deal of arene derivatives are prepared using them. With the aid of the Sandmeyer reaction, we are able to accomplish special transformations on benzene, such as halogenation, cyanation, and hydroxylation.
Complete step-by-step answer:We must explain how the Sandmeyer reaction will be used to produce chlorobenzene from benzene diazonium chloride in order to answer the question.
As a result, aryl halides are produced from aryl diazonium salts using the Sandmeyer reaction, a chemical process. Traugott Sandmeyer, a Swiss chemist, is honored by the name of this reaction. The Sandmeyer reaction, which is frequently catalysed by copper \[(I)\] salts that is a procedure for the substituting an aromatic amino group by preparing its diazonium salt which is followed by its displacement. It is typically catalysed by copper salts. The nucleophile might be anything from cyanide to thiols to water to halide anions.
When cuprous chloride or cuprous bromide are applied to diazonium salt, respectively, chlorobenzene or bromobenzene is generated.

Option ‘A’ is correct
Note: It should be noted that an aromatic primary amine is processed with \[NaN{O_2}\] and dilute\[HCl\] at low temperature to produce benzene diazonium salt. The action is referred to as diazotization. The chemicals called diazonium salts are very reactive. A great deal of arene derivatives are prepared using them. With the aid of the Sandmeyer reaction, we are able to accomplish special transformations on benzene, such as halogenation, cyanation, and hydroxylation.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




