
Nitrene is an intermediate in one of the following reactions:
A. Schmidt rearrangement
B. Beckmann rearrangement
C. Baeyer-Villiger oxidation
D. Curtius reaction
Answer
241.2k+ views
Hint : Nitrene is the nitrogen analogue of a carbene. It is an electrophile because it has five valence electrons. It is involved in many chemical reactions and acts as a reactive intermediate. It can be generated by two common ways. It can be generated by thermolysis or photolysis of azides with expulsion of nitrogen gas. Another way of generation of nitrene is the expulsion of carbon monoxide.
Complete step by step solution:
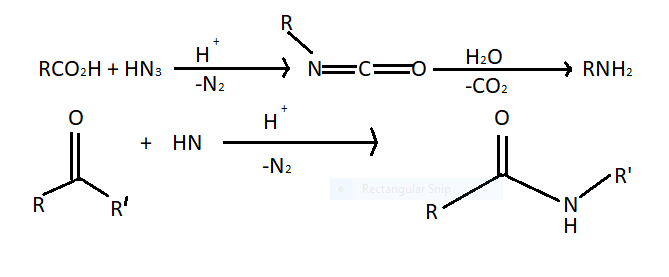
In Schmidt rearrangement alkyl migration over carbon nitrogen bonds takes place. This reaction refers to a reaction between an acid catalyst hydrazoic acid with electrophiles. Carboxylic acid forms amines through an isocyanate intermediate and ketones from amides. Nitrene is an intermediate in the following reactions.
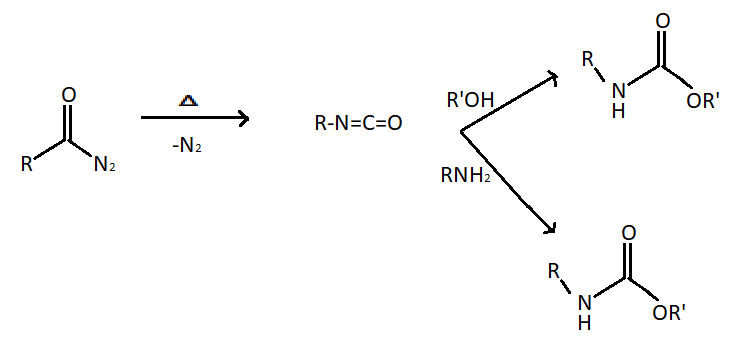
In the Curtius reaction, the production of an isocyanate is accomplished by the thermal decomposition of carboxylic azides. The mechanism involves the alkyl shift of the $R$ group from carbonyl carbon to the closet nitrogen. The reaction has similarities with Schmidt reaction with acids but differs in that the acyl azide in the present case is prepared from acyl halide and an azide salt.
Hence option A and D both are correct answers of this problem because in both of the reactions nitrene is an intermediate.
Note : Nitrenes are highly reactive molecule species with a monovalent nitrogen atom which can exist in a singlet and triplet state. Nitrenes are more stable than carbene. It is because of greater thermodynamic stability of nitrene. As nitrenes are very reactive they are not isolated. The insertion of nitrene can be easily into a carbon to hydrogen covalent bond yielding an amine or amide.
Complete step by step solution:
In Schmidt rearrangement alkyl migration over carbon nitrogen bonds takes place. This reaction refers to a reaction between an acid catalyst hydrazoic acid with electrophiles. Carboxylic acid forms amines through an isocyanate intermediate and ketones from amides. Nitrene is an intermediate in the following reactions.

In the Curtius reaction, the production of an isocyanate is accomplished by the thermal decomposition of carboxylic azides. The mechanism involves the alkyl shift of the $R$ group from carbonyl carbon to the closet nitrogen. The reaction has similarities with Schmidt reaction with acids but differs in that the acyl azide in the present case is prepared from acyl halide and an azide salt.

Hence option A and D both are correct answers of this problem because in both of the reactions nitrene is an intermediate.
Note : Nitrenes are highly reactive molecule species with a monovalent nitrogen atom which can exist in a singlet and triplet state. Nitrenes are more stable than carbene. It is because of greater thermodynamic stability of nitrene. As nitrenes are very reactive they are not isolated. The insertion of nitrene can be easily into a carbon to hydrogen covalent bond yielding an amine or amide.
Recently Updated Pages
Know The Difference Between Fluid And Liquid

Difference Between Crystalline and Amorphous Solid: Table & Examples

Types of Solutions in Chemistry: Explained Simply

Hess Law of Constant Heat Summation: Definition, Formula & Applications

Disproportionation Reaction: Definition, Example & JEE Guide

JEE Extractive Metallurgy Important Concepts and Tips for Exam Preparation

Trending doubts
JEE Main 2026: Session 1 Results Out and Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

Ideal and Non-Ideal Solutions Explained for Class 12 Chemistry

JEE Main Participating Colleges 2026 - A Complete List of Top Colleges

Clemmensen and Wolff Kishner Reductions Explained for JEE & NEET

Degree of Dissociation: Meaning, Formula, Calculation & Uses

Understanding the Angle of Deviation in a Prism

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

JEE Advanced 2026 - Exam Date (Released), Syllabus, Registration, Eligibility, Preparation, and More

CBSE Notes Class 11 Chemistry Chapter 9 - Hydrocarbons - 2025-26

CBSE Notes Class 11 Chemistry Chapter 5 - Thermodynamics - 2025-26

CBSE Notes Class 11 Chemistry Chapter 6 - Equilibrium - 2025-26

Inductive Effect and Its Role in Acidic Strength




