
In which thermodynamic process, volume remains same?
A. Isobaric
B. Isothermal
C. Adiabatic
D. Isochoric
Answer
232.8k+ views
Hint: Let's first understand the concept of thermodynamic process fundamentals in order to answer this question. We know that $P{V^r} = {\text{constant}}$ because of the ideal gas law. Thus $PV = nRT = {\text{constant}}$. This means that for a certain sample of gas, the product of a gas's pressure and volume is constant.
Complete answer:
Let us consider all the four options given one by one: -
1. The isobaric process in thermodynamics is a process during which the pressure of a system remains constant that’s why it is also referred to as a constant-pressure process. $\Delta P = 0$
2. The isothermal process, also known as a constant-temperature process, is a thermodynamic process in which the temperature of a system remains constant $\Delta T = 0$.
3. The adiabatic process in thermodynamics is a process during which there is no heat exchange with the system $P{V^r} = {\text{constant}}$.
4. The isochoric process in thermodynamics is a process during which the volume of a system remains constant that’s why it is also referred to as a constant-volume process $\Delta V = 0$.
5. The system isolates itself from its surroundings by a process known as adiabatic. A thermodynamic system and its environment engage in an adiabatic process when neither heat nor mass of any kind is transferred. Insulation from the environment is, therefore, necessary for the adiabatic process, which is true.
Graphically, an isochoric process can be represented as: -
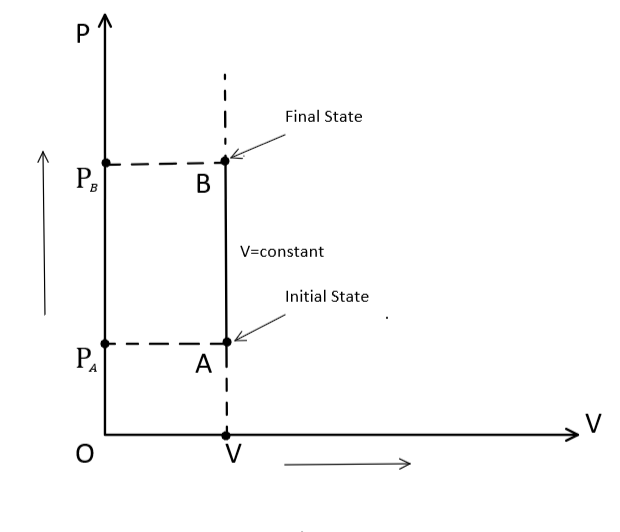
Thus, in thermodynamics, volume remains the same in the Isochoric process. Hence, the correct option is (D) Isochoric.
Note: As we know, an isobaric process is one in which the system's pressure remains constant. And when a system's macroscopic attributes, such as temperature and pressure, do not fluctuate over time, it is said to be in thermodynamic equilibrium.
Complete answer:
Let us consider all the four options given one by one: -
1. The isobaric process in thermodynamics is a process during which the pressure of a system remains constant that’s why it is also referred to as a constant-pressure process. $\Delta P = 0$
2. The isothermal process, also known as a constant-temperature process, is a thermodynamic process in which the temperature of a system remains constant $\Delta T = 0$.
3. The adiabatic process in thermodynamics is a process during which there is no heat exchange with the system $P{V^r} = {\text{constant}}$.
4. The isochoric process in thermodynamics is a process during which the volume of a system remains constant that’s why it is also referred to as a constant-volume process $\Delta V = 0$.
5. The system isolates itself from its surroundings by a process known as adiabatic. A thermodynamic system and its environment engage in an adiabatic process when neither heat nor mass of any kind is transferred. Insulation from the environment is, therefore, necessary for the adiabatic process, which is true.
Graphically, an isochoric process can be represented as: -

Thus, in thermodynamics, volume remains the same in the Isochoric process. Hence, the correct option is (D) Isochoric.
Note: As we know, an isobaric process is one in which the system's pressure remains constant. And when a system's macroscopic attributes, such as temperature and pressure, do not fluctuate over time, it is said to be in thermodynamic equilibrium.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Laws of Motion Class 11 Physics Chapter 4 CBSE Notes - 2025-26

Waves Class 11 Physics Chapter 14 CBSE Notes - 2025-26

Mechanical Properties of Fluids Class 11 Physics Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Physics Chapter 11 CBSE Notes - 2025-26

Units And Measurements Class 11 Physics Chapter 1 CBSE Notes - 2025-26




