
In the following reaction the catalyst used is-
A. Al2O3
B. Cr2O3
C. Al2O3 & Cr2O3
D. Zn dust
Answer
232.8k+ views
Hint: In most of the oxidation reactions in organic chemistry Cr2O3 is used to oxidize the compounds and also for cyclization. Cr2O3 is a strong oxidizing agent. Al2O3 is also a good oxidizing agent and also a dehydrogenation catalyst. It removes H2 molecules from the compounds. Together they act as a good oxidizing agent and as well as used as a catalyst for aromatization. The conversion of aliphatic organic compounds into aromatic compounds is called aromatization. Aromatic compounds are more stable than aliphatic compounds.
Complete Step by Step Answer:
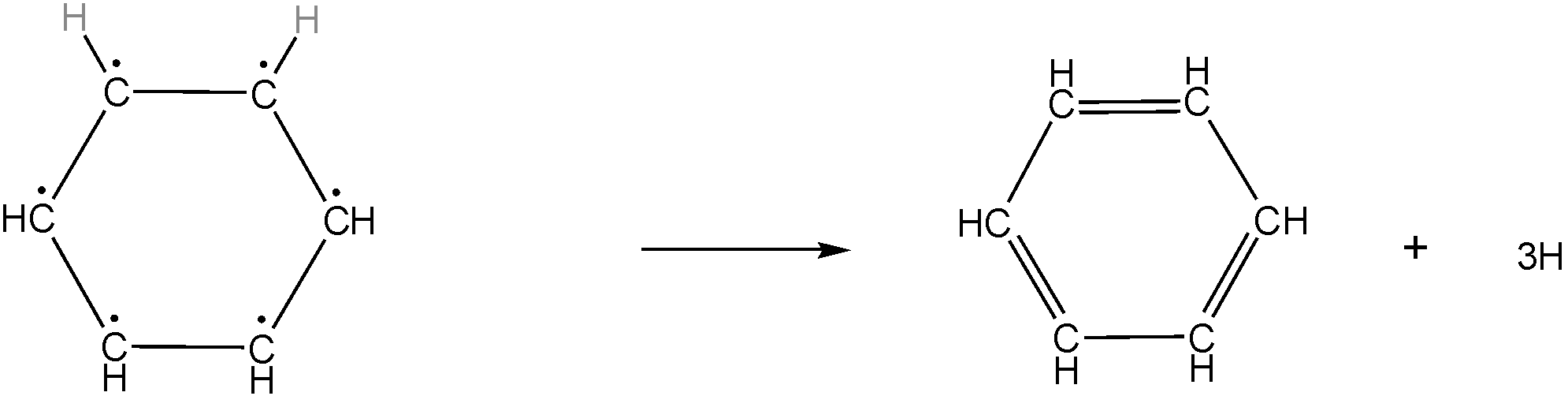
Given reaction in the question is-
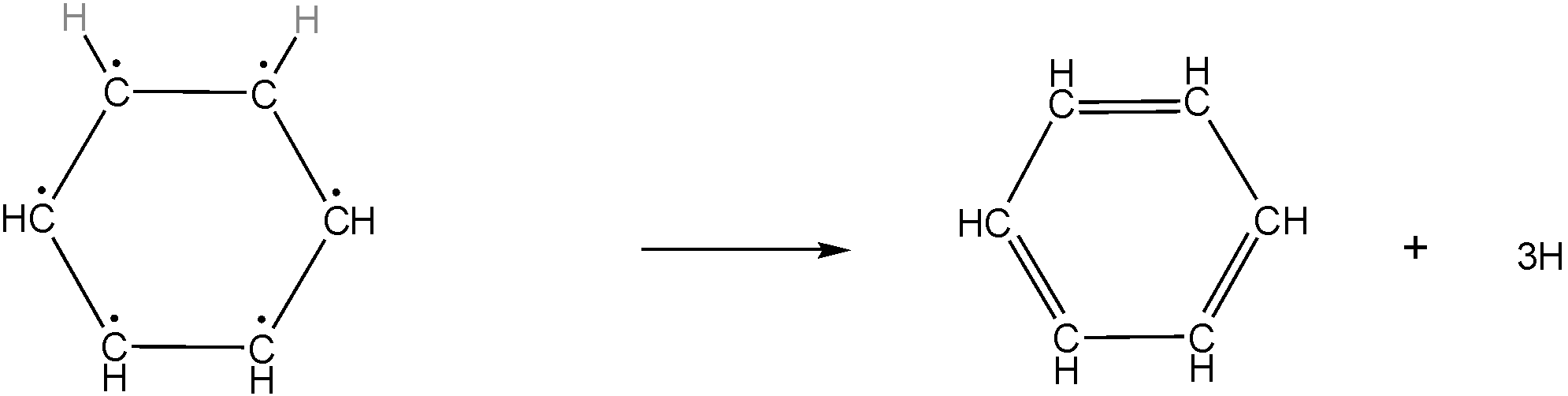
This chemical reaction is practically, chemically, or thermodynamically not possible because the reactant in this reaction contains 6 radical carbon which is a highly unstable compound so it cannot be formed even as an intermediate in the reaction.
But we can convert n-Hexane into benzene using Al2O3 & Cr2O3 as catalyst because Cr2O3 & Al2O3 acts as a good oxidizing agent and good dehydrogenating agent respectively. So together in combination with each other, they act as a good catalyst for cyclization & aromatization in organic chemistry. It is also called a reforming process.
The given reaction will be:
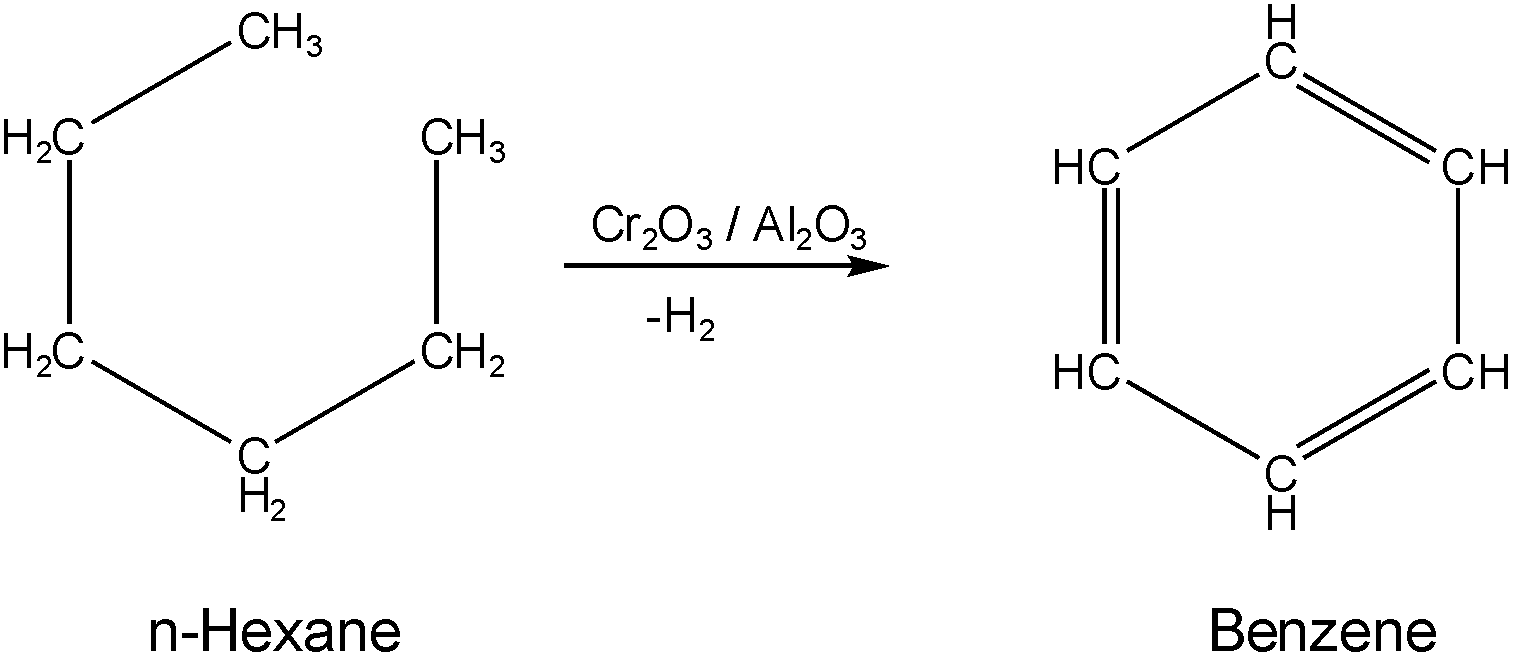
And the mechanism of the reaction is
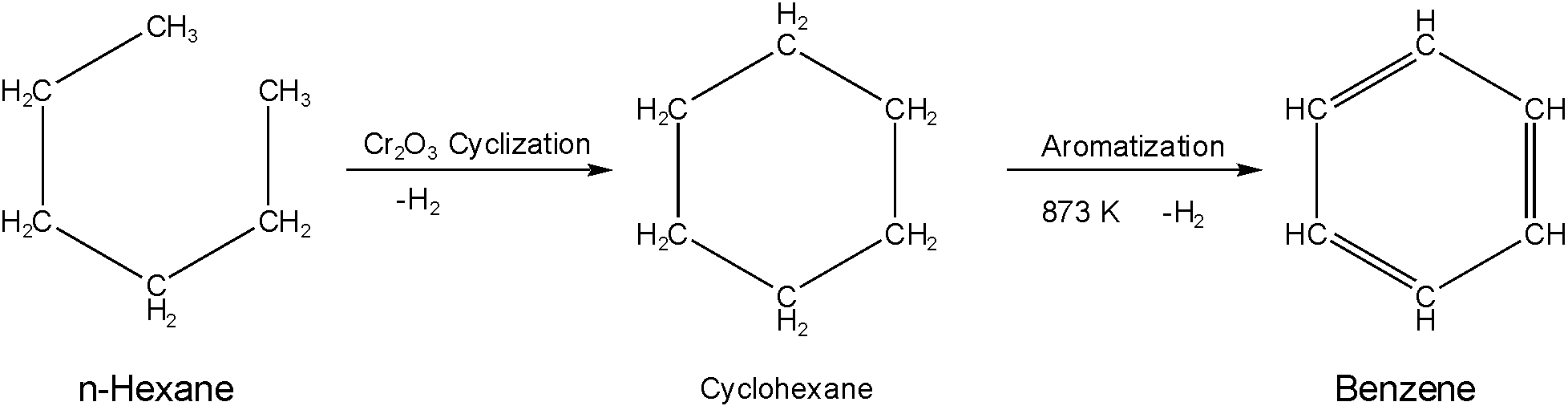
In the reaction, the terminal carbons oxidized in presence of Cr2O3 and form a covalent bond converting n-hexane into cyclohexane. Then in presence of Al2O3, it aromatizes and forms benzene.
Thus, Option (C) is correct
Note: Vanadium oxide is also used for the aromatization of benzene. To produce toluene from n-hexane we can use Friedel Craft Alkylation. This aromatization of n-hexane is also called dehydroaromatization.
Complete Step by Step Answer:
Given reaction in the question is-

This chemical reaction is practically, chemically, or thermodynamically not possible because the reactant in this reaction contains 6 radical carbon which is a highly unstable compound so it cannot be formed even as an intermediate in the reaction.
But we can convert n-Hexane into benzene using Al2O3 & Cr2O3 as catalyst because Cr2O3 & Al2O3 acts as a good oxidizing agent and good dehydrogenating agent respectively. So together in combination with each other, they act as a good catalyst for cyclization & aromatization in organic chemistry. It is also called a reforming process.
The given reaction will be:

And the mechanism of the reaction is

In the reaction, the terminal carbons oxidized in presence of Cr2O3 and form a covalent bond converting n-hexane into cyclohexane. Then in presence of Al2O3, it aromatizes and forms benzene.
Thus, Option (C) is correct
Note: Vanadium oxide is also used for the aromatization of benzene. To produce toluene from n-hexane we can use Friedel Craft Alkylation. This aromatization of n-hexane is also called dehydroaromatization.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




