
Identify X and Y in the following sequence

A.\[{\rm{X = KCN,Y = LiAl}}{{\rm{H}}_{\rm{4}}}\]
B. \[{\rm{X = KCN,Y = }}{{\rm{H}}_{\rm{3}}}{{\rm{O}}^{\rm{ + }}}\]
C. \[{\rm{X = C}}{{\rm{H}}_{\rm{3}}}{\rm{Cl,Y = AlC}}{{\rm{l}}_{\rm{3}}}{\rm{/HCl}}\]
D. \[{\rm{X = C}}{{\rm{H}}_{\rm{3}}}{\rm{N}}{{\rm{H}}_{\rm{2}}}{\rm{,Y = HN}}{{\rm{O}}_{\rm{2}}}\]
Answer
233.1k+ views
Hint: Alkyl halides on reaction with alcoholic potassium cyanide or sodium cyanide form alkyl cyanide. Alkyl cyanide on reduction with lithium aluminium hydride forms primary amines.
Complete Step by Step Solution:
Amines are formed by the replacement of one, two or all three hydrogen atoms of ammonia by alkyl or aryl groups.
A. Bromoethane in reaction with alcoholic potassium cyanide forms propionitrile.
Propionitrile then on reaction lithium aluminium hydride undergoes reduction.
It forms the primary amine having one carbon atom more than the parent ethyl bromide.
Thus, it forms propan-1-amine as the product.
In the given reaction, the last product formed is also propan-1-amine.
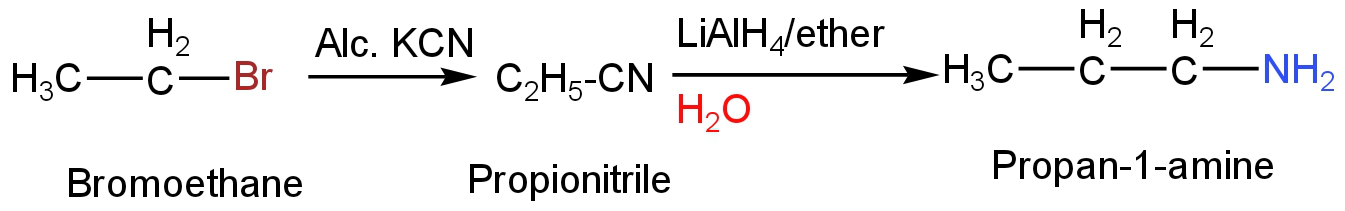
Image: Reaction of bromoethane with KCN.
So, option A is correct.
B. Bromoethane on reaction with KCN will form propionitrile.
Propionitrile in presence of hydronium ion will undergo acid hydrolysis to form propionic acid.
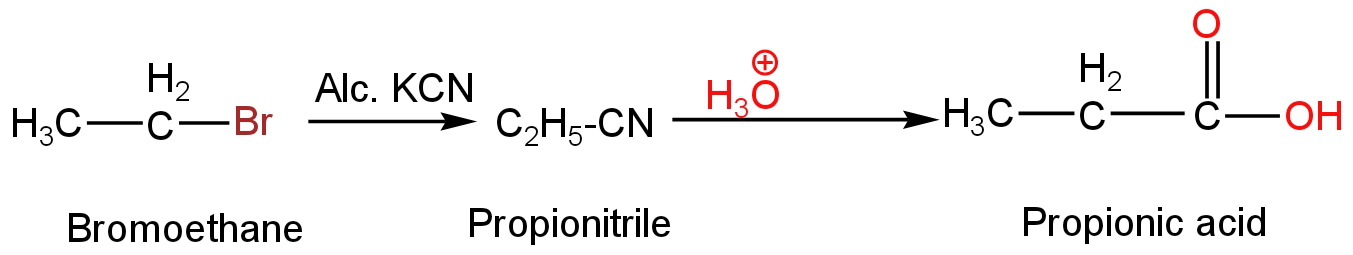
Image: Reaction of propionitrile with hydronium ion.
So, it will not form propan-1-amine.
Thus, option B is incorrect.
C. Bromoethane and chloromethane will not react with each other. So, this reaction will not happen.
So, option C is incorrect.
D. Bromoethane on reaction with ethanenitrile will form N-Methylethanamine and hydrogen bromide.
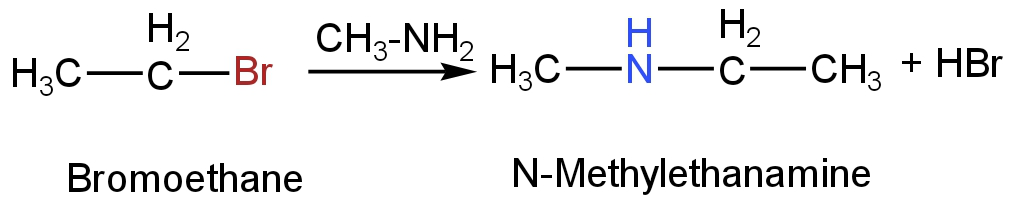
Image: Reaction of bromoethane with ethanenitrile.
N-Methylethanamine will not react with nitrous acid. It is because nitrous acid reacts with\[{\rm{R - N}}{{\rm{H}}_{\rm{2}}}{\rm{orAr - N}}{{\rm{H}}_{\rm{2}}}\]to form alkyl diazonium chloride or aryl diazonium chloride i.e., \[{\rm{R - N}}_2^ + {\rm{ClorAr - N}}_2^ + {\rm{C}}{{\rm{l}}^{\rm{ - }}}\].
But in N-Methylethanamine, there are only two hydrogen atoms attached to Nitrogen atoms.
So, option D is incorrect.
So, option A is correct.
Note: Bromoethane reacts with KCN to form propionitrile. Propionitrile on reaction with lithium aluminium hydride undergoes reduction to form propan-1-amine. Propan-1-amine has one carbon more than bromoethane. While attending to the question, one must choose the option in which the end product is propan-1-amine.
Complete Step by Step Solution:
Amines are formed by the replacement of one, two or all three hydrogen atoms of ammonia by alkyl or aryl groups.
A. Bromoethane in reaction with alcoholic potassium cyanide forms propionitrile.
Propionitrile then on reaction lithium aluminium hydride undergoes reduction.
It forms the primary amine having one carbon atom more than the parent ethyl bromide.
Thus, it forms propan-1-amine as the product.
In the given reaction, the last product formed is also propan-1-amine.

Image: Reaction of bromoethane with KCN.
So, option A is correct.
B. Bromoethane on reaction with KCN will form propionitrile.
Propionitrile in presence of hydronium ion will undergo acid hydrolysis to form propionic acid.

Image: Reaction of propionitrile with hydronium ion.
So, it will not form propan-1-amine.
Thus, option B is incorrect.
C. Bromoethane and chloromethane will not react with each other. So, this reaction will not happen.
So, option C is incorrect.
D. Bromoethane on reaction with ethanenitrile will form N-Methylethanamine and hydrogen bromide.

Image: Reaction of bromoethane with ethanenitrile.
N-Methylethanamine will not react with nitrous acid. It is because nitrous acid reacts with\[{\rm{R - N}}{{\rm{H}}_{\rm{2}}}{\rm{orAr - N}}{{\rm{H}}_{\rm{2}}}\]to form alkyl diazonium chloride or aryl diazonium chloride i.e., \[{\rm{R - N}}_2^ + {\rm{ClorAr - N}}_2^ + {\rm{C}}{{\rm{l}}^{\rm{ - }}}\].
But in N-Methylethanamine, there are only two hydrogen atoms attached to Nitrogen atoms.
So, option D is incorrect.
So, option A is correct.
Note: Bromoethane reacts with KCN to form propionitrile. Propionitrile on reaction with lithium aluminium hydride undergoes reduction to form propan-1-amine. Propan-1-amine has one carbon more than bromoethane. While attending to the question, one must choose the option in which the end product is propan-1-amine.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




