
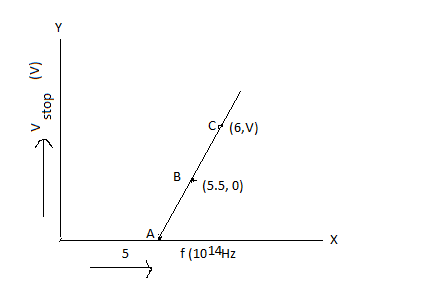
Given figure shows few data points in a photo-electric experiment for a certain metal. The minimum energy for ejection of electron from its surface is: (Planck’s constant \[h = 6.62 \times {10^{ - 34}}\,{\rm{J}}{\rm{.s}}\] )
A. 2.10 eV
B. 2.27 eV
C. 2.59 eV
D. 1.93 eV
Answer
232.8k+ views
Hint: The electrons get emitted from the metallic surface on exposing it to a suitable frequency light. This phenomenon is termed photoelectric effect. Some light energies such as UV rays, X rays, Infrared rays etc. cause photoelectric effect.
Formula used:
Formula of threshold energy \[\varphi \] is,
\[\varphi = h{f_0}\]
Where, h is Planck’s constant and \[{f_0}\] is frequency.
Complete Step by Step Solution:
Let’s understand threshold energy first. The threshold energy means the minimum requirement of energy for a reaction to undergo.
In the given question, a graph of frequency and stopping potential is shown for the photoelectric effect of the metal. Now, we have to calculate the threshold energy using the values given in the graph.
We should consider the frequency of point B, to calculate the threshold energy of the metal. Because at this point, stopping potential is zero.
So, \[f = 5.5 \times {10^{14}}\,{\rm{Hz}}\] and \[h = 6.62 \times {10^{ - 34}}\,{\rm{J}}{\rm{.s}}\].
Now, Putting the value of f and h in the threshold energy formula, that is,\[\varphi = h{f_0}\]
\[\varphi = 6.62 \times {10^{ - 34}}\,{\rm{J}}{\rm{.s}} \times 5.5 \times {10^{14}}\,{\rm{Hz}}\]
\[\varphi \,{\rm{ = }}\;{\rm{36}}{\rm{.41}} \times {\rm{1}}{{\rm{0}}^{ - 20}}\,{\rm{Hz}}\]
Now, we have to convert energy from Hertz to electron volt.
1 electron volt=\[1.6021 \times {10^{ - 19}}\,{\rm{J}}\]
So, \[\varphi \,{\rm{ = }}\;\dfrac{{{\rm{36}}{\rm{.41}} \times {\rm{1}}{{\rm{0}}^{ - 20}}\,}}{{1.6021 \times {{10}^{ - 19}}}} = 22.73 \times {10^{ - 1}} = 2.27\,\,{\rm{eV}}\]
Therefore, the correct threshold energy is 2.27 eV. Hence, the correct option is B.
Note: It is to be noted that photoelectric effect is also sometimes shown by non-metals. To a limited extent, gases and liquids also show this effect. The emitted electrons due to the photoelectric effect is known as photoelectrons.
Formula used:
Formula of threshold energy \[\varphi \] is,
\[\varphi = h{f_0}\]
Where, h is Planck’s constant and \[{f_0}\] is frequency.
Complete Step by Step Solution:
Let’s understand threshold energy first. The threshold energy means the minimum requirement of energy for a reaction to undergo.
In the given question, a graph of frequency and stopping potential is shown for the photoelectric effect of the metal. Now, we have to calculate the threshold energy using the values given in the graph.
We should consider the frequency of point B, to calculate the threshold energy of the metal. Because at this point, stopping potential is zero.
So, \[f = 5.5 \times {10^{14}}\,{\rm{Hz}}\] and \[h = 6.62 \times {10^{ - 34}}\,{\rm{J}}{\rm{.s}}\].
Now, Putting the value of f and h in the threshold energy formula, that is,\[\varphi = h{f_0}\]
\[\varphi = 6.62 \times {10^{ - 34}}\,{\rm{J}}{\rm{.s}} \times 5.5 \times {10^{14}}\,{\rm{Hz}}\]
\[\varphi \,{\rm{ = }}\;{\rm{36}}{\rm{.41}} \times {\rm{1}}{{\rm{0}}^{ - 20}}\,{\rm{Hz}}\]
Now, we have to convert energy from Hertz to electron volt.
1 electron volt=\[1.6021 \times {10^{ - 19}}\,{\rm{J}}\]
So, \[\varphi \,{\rm{ = }}\;\dfrac{{{\rm{36}}{\rm{.41}} \times {\rm{1}}{{\rm{0}}^{ - 20}}\,}}{{1.6021 \times {{10}^{ - 19}}}} = 22.73 \times {10^{ - 1}} = 2.27\,\,{\rm{eV}}\]
Therefore, the correct threshold energy is 2.27 eV. Hence, the correct option is B.
Note: It is to be noted that photoelectric effect is also sometimes shown by non-metals. To a limited extent, gases and liquids also show this effect. The emitted electrons due to the photoelectric effect is known as photoelectrons.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




