
Gem-dibromide is
A. \[C{H_3}CB{r_2}C{H_3}\]
B. \[C{H_2}BrC{H_2}Br\]
C. \[C{H_2}(Br)C{H_2}C{H_2}\]
D. None of these
Answer
233.1k+ views
Hint: The connection between the two atoms or the functional groups that is attached to the same atom, it is referred to as a "gem.". The Latin root of the word gem means "twins." Methan-diol is an example of a gem diol which is a molecule that having the two alcohol functional groups connected to the same carbon atom. Additionally, the shorter prefix gem may be used to indicate this connection before a chemical name, as in a gem-dibromide.
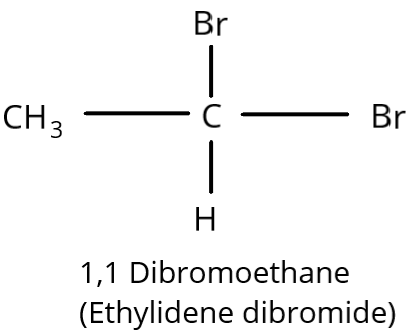
Complete step-by-step answer:In order to know that the two halide groups must be situated on the same carbon atom naming as \[1,1\] connection for geminal dihalides also exist.
\[1,1\] Dibromoethane is another name for Gem-ethyl dibromide. It has a \[1,1\] connection because it has two bromine atoms on a single carbon atom. It is hence geminal dihalide.
In short, when the two functional groups or atoms that are attached with the same carbon atom, this condition is called geminal dihalide.
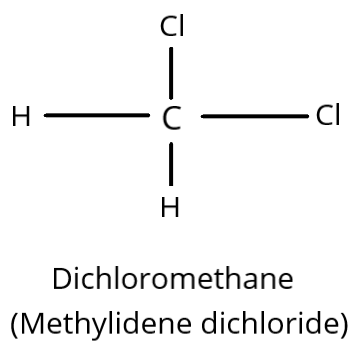
Dichloromethane is another name for methylidene dichloride. It has a connection because it has two Cl-atoms attached to the same carbon atom. It is hence geminal dihalide.
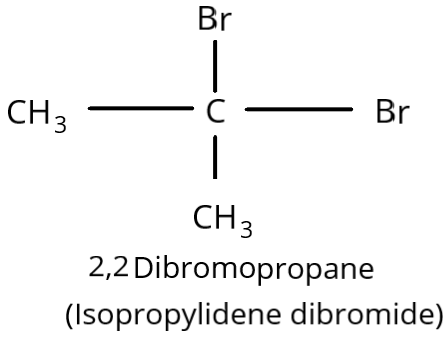
\[2,2\] Dibromopropane(\[C{H_3}CB{r_2}C{H_3}\]) is another name for isopropylidene dibromide. It has a \[2,2\] connection because it has two bromine atoms on the same carbon atom. It is hence geminal dihalide.
Option ‘A’ is correct
Note: It should be noted that the term "geminal" describes the bond between two atoms or functional groups that are joined to the same atom. A gem-dibromide, which stands for "geminal dibromide," is another way to denote this link before a chemical name. Because functional groups that are connected to the same atom frequently behave differently than when they are separated, synthesis and spectroscopy are being included. For instance, geminal diols readily undergo the water-loss conversion to ketones or aldehydes.
Complete step-by-step answer:In order to know that the two halide groups must be situated on the same carbon atom naming as \[1,1\] connection for geminal dihalides also exist.
\[1,1\] Dibromoethane is another name for Gem-ethyl dibromide. It has a \[1,1\] connection because it has two bromine atoms on a single carbon atom. It is hence geminal dihalide.
In short, when the two functional groups or atoms that are attached with the same carbon atom, this condition is called geminal dihalide.

Dichloromethane is another name for methylidene dichloride. It has a connection because it has two Cl-atoms attached to the same carbon atom. It is hence geminal dihalide.

\[2,2\] Dibromopropane(\[C{H_3}CB{r_2}C{H_3}\]) is another name for isopropylidene dibromide. It has a \[2,2\] connection because it has two bromine atoms on the same carbon atom. It is hence geminal dihalide.

Option ‘A’ is correct
Note: It should be noted that the term "geminal" describes the bond between two atoms or functional groups that are joined to the same atom. A gem-dibromide, which stands for "geminal dibromide," is another way to denote this link before a chemical name. Because functional groups that are connected to the same atom frequently behave differently than when they are separated, synthesis and spectroscopy are being included. For instance, geminal diols readily undergo the water-loss conversion to ketones or aldehydes.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




