
Correct statement about but-1,3-diene
A. Conjugated double bonds are present
B. Reacts with HBr
C. Forms polymer
D. All of these
Answer
232.8k+ views
Hint: But-1,3-diene has two pi-bonds. It can be regarded as the combination of two vinyl groups. Polybutadiene rubber is a polymer made by the polymerization of this compound.
Complete Step by Step Solution:
Hydrocarbons having two double bonds are called dienes.
Dienes are classified into three types depending on the location of double bonds.
1. Isolated dienes
2. Cumulated dienes
C. Conjugated dienes
But-1,3-diene is a conjugated diene. A single bond intervenes in two double bonds i.e., double bonds and single bonds are alternately arranged.
Its structure is as follows:
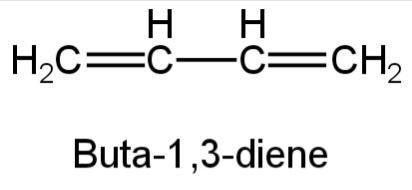
Image: 1,3-butadiene
So, A is correct.
The addition of HBr to 1,3-butadiene gives a mixture of 1,2 and 1,4 addition products.
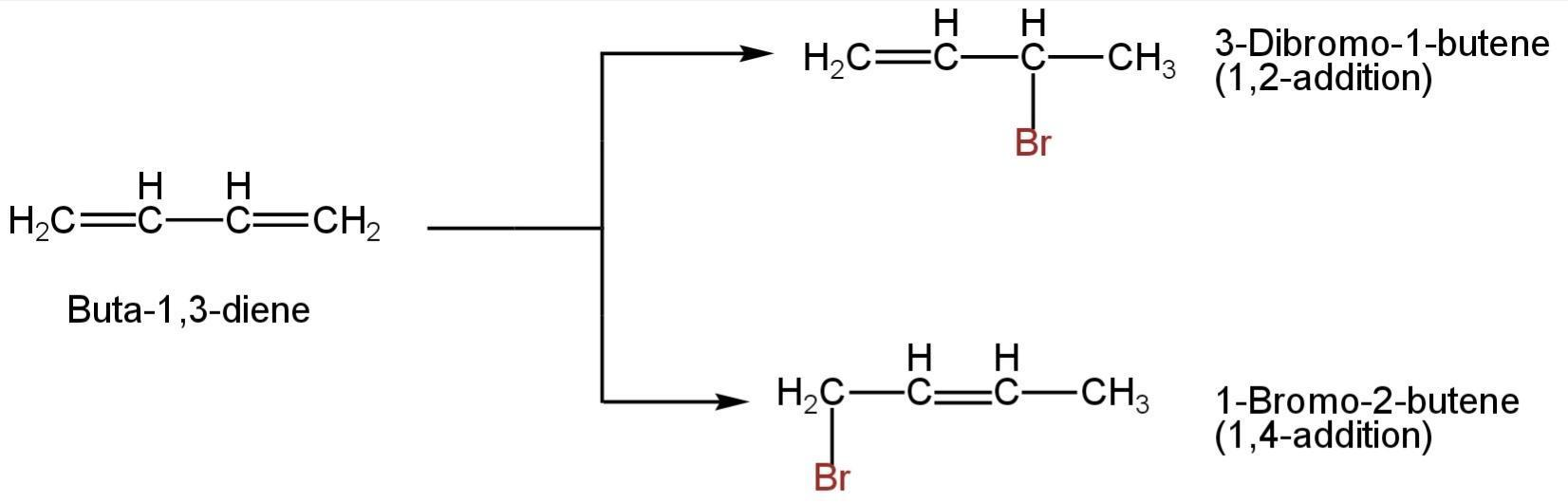
Image: Addition of HBr to 1,3-butadiene
The mechanism is as follows:
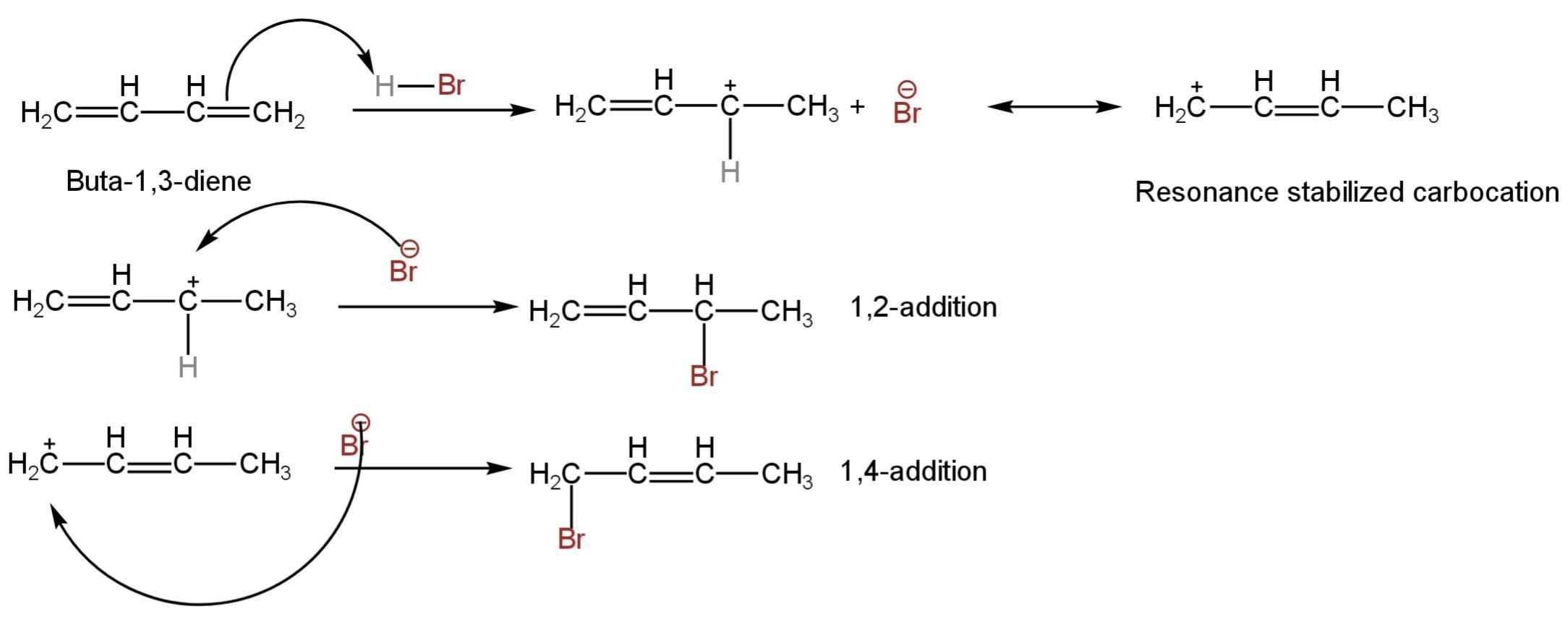
Image: Mechanism of addition of HBr to 1,3-butadiene.
So, B is correct.
Polybutadiene rubber is a polymer created by the polymerization of this compound.
It is a synthetic rubber.
It is a polymer created from the polymerization of the monomer 1,3-butadiene.
The reaction happens as follows:
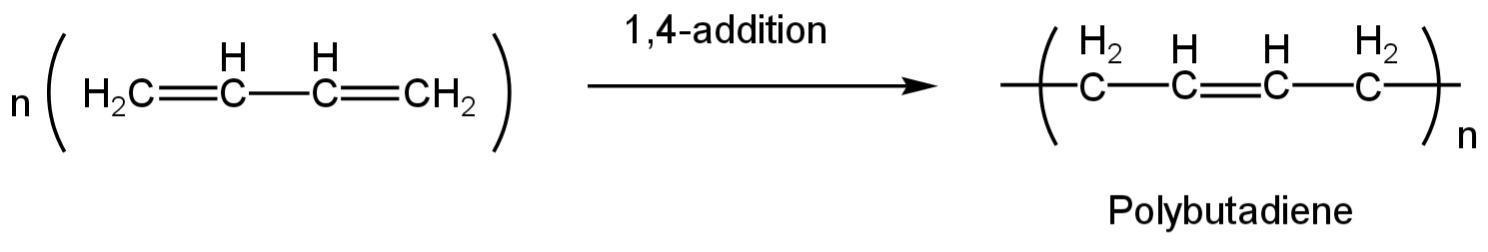
Image: Polymerization of 1,3-butadiene
So, C is correct.
As A, B and C are correct. So, D is correct.
So, option D is correct.
Note: The transformation of butadiene to synthetic rubbers is polymerization. It is a process by which small molecules or monomers are attached to give rise to large molecules called polymers leading to the formation of polybutadiene, which is very soft rubber.
Complete Step by Step Solution:
Hydrocarbons having two double bonds are called dienes.
Dienes are classified into three types depending on the location of double bonds.
1. Isolated dienes
2. Cumulated dienes
C. Conjugated dienes
But-1,3-diene is a conjugated diene. A single bond intervenes in two double bonds i.e., double bonds and single bonds are alternately arranged.
Its structure is as follows:

Image: 1,3-butadiene
So, A is correct.
The addition of HBr to 1,3-butadiene gives a mixture of 1,2 and 1,4 addition products.

Image: Addition of HBr to 1,3-butadiene
The mechanism is as follows:

Image: Mechanism of addition of HBr to 1,3-butadiene.
So, B is correct.
Polybutadiene rubber is a polymer created by the polymerization of this compound.
It is a synthetic rubber.
It is a polymer created from the polymerization of the monomer 1,3-butadiene.
The reaction happens as follows:

Image: Polymerization of 1,3-butadiene
So, C is correct.
As A, B and C are correct. So, D is correct.
So, option D is correct.
Note: The transformation of butadiene to synthetic rubbers is polymerization. It is a process by which small molecules or monomers are attached to give rise to large molecules called polymers leading to the formation of polybutadiene, which is very soft rubber.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




