
Consider the following reaction,\[X\xrightarrow{{Bromination}}Y\xrightarrow[{ + HCl}]{{NaN{O_2}}}Z\xrightarrow[{{C_2}{H_5}OH}]{{Boiling}}\]Tribromo-benzene. \[X\]is
(A) Benzoic acid
(B) Salicylic acid
(C) Phenol
(D) Aniline
Answer
232.8k+ views
Hint: Organic substance aniline has the chemical formula \[{C_6}{H_5}N{H_2}\]. The simplest aromatic amine is aniline, which consists of an amino group joined to a phenyl group. It is an important commodity chemical for industry and a flexible starting point for the synthesis of fine chemicals. The production of colours, industrial chemicals, and precursors to polyurethane are its principal uses.
Complete Step by Step Solution:
In the question, we have given the reaction \[X\xrightarrow{{Bromination}}Y\xrightarrow[{ + HCl}]{{NaN{O_2}}}Z\xrightarrow[{{C_2}{H_5}OH}]{{Boiling}}\],
In order to know that by using the gas chromatography-mass spectrometry to determine the amount of cyanide in human plasma and urine, tribromo-benzene serves as an internal standard during the derivatization process. And it joins in stoichiometric \[1:2\] molecular complexes with fullerenes.
Let’s notice the process of the conversion of tribromo-aniline to tribromo-benzene:
Let’s start by the bromination of aniline: Aniline is brominated when being in the presence of aqueous \[B{r_2}\].
The reaction is as follow:
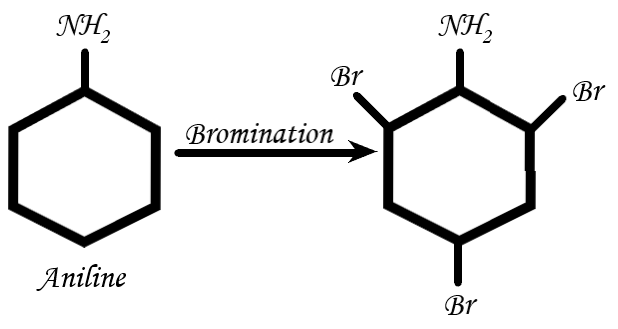
(Self made)
Now, let’s consider the second step: Diazotization is carried in the presence of \[NaN{O_2} + HCl\]. The reaction is as follows:
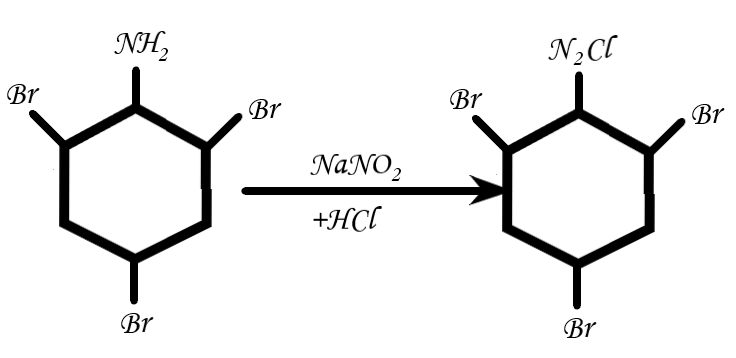
(Self made)
Then, notice the last step which is reduction with \[{C_2}{H_5}OH\]: When the diazonium salt is reduced with \[{C_2}{H_5}OH\]then tribromo-benzene is produced.
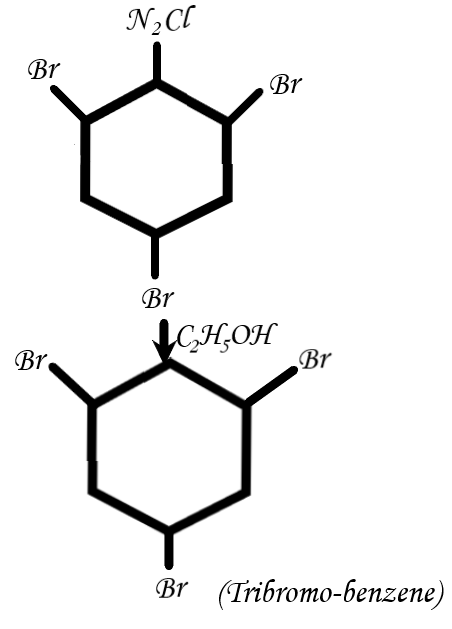
(Self made)
Therefore, \[X\]is aniline in the reaction \[X\xrightarrow{{Bromination}}Y\xrightarrow[{ + HCl}]{{NaN{O_2}}}Z\xrightarrow[{{C_2}{H_5}OH}]{{Boiling}}\]Tribromo-benzene.
Thus, the correct option is: (D) Aniline.
Note: It should be noted that the tribromo-benzene is a crucial step in organic synthesis that is mostly used in the creation of pharmaceuticals. The boiling and fusing temperatures of the tribromo-benzene are \[124^\circ C\]and \[271^\circ C\], respectively. It is water insoluble and that can be dissolved in a hot ethanol. In the current preparation, the procedure for producing the tribromo-benzene involves adding ethanol to a reaction flask containing copper sulphate, stirring, and adding vitriol oil. This mixture is then heated in a water bath and treated by adding \[NaNO\]after the raw materials have all dissolved to boiling in batches to finish.
Complete Step by Step Solution:
In the question, we have given the reaction \[X\xrightarrow{{Bromination}}Y\xrightarrow[{ + HCl}]{{NaN{O_2}}}Z\xrightarrow[{{C_2}{H_5}OH}]{{Boiling}}\],
In order to know that by using the gas chromatography-mass spectrometry to determine the amount of cyanide in human plasma and urine, tribromo-benzene serves as an internal standard during the derivatization process. And it joins in stoichiometric \[1:2\] molecular complexes with fullerenes.
Let’s notice the process of the conversion of tribromo-aniline to tribromo-benzene:
Let’s start by the bromination of aniline: Aniline is brominated when being in the presence of aqueous \[B{r_2}\].
The reaction is as follow:

(Self made)
Now, let’s consider the second step: Diazotization is carried in the presence of \[NaN{O_2} + HCl\]. The reaction is as follows:

(Self made)
Then, notice the last step which is reduction with \[{C_2}{H_5}OH\]: When the diazonium salt is reduced with \[{C_2}{H_5}OH\]then tribromo-benzene is produced.

(Self made)
Therefore, \[X\]is aniline in the reaction \[X\xrightarrow{{Bromination}}Y\xrightarrow[{ + HCl}]{{NaN{O_2}}}Z\xrightarrow[{{C_2}{H_5}OH}]{{Boiling}}\]Tribromo-benzene.
Thus, the correct option is: (D) Aniline.
Note: It should be noted that the tribromo-benzene is a crucial step in organic synthesis that is mostly used in the creation of pharmaceuticals. The boiling and fusing temperatures of the tribromo-benzene are \[124^\circ C\]and \[271^\circ C\], respectively. It is water insoluble and that can be dissolved in a hot ethanol. In the current preparation, the procedure for producing the tribromo-benzene involves adding ethanol to a reaction flask containing copper sulphate, stirring, and adding vitriol oil. This mixture is then heated in a water bath and treated by adding \[NaNO\]after the raw materials have all dissolved to boiling in batches to finish.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




