
Consider the following anions.
When attached to $s{p^{3 - }}$ hybridised carbon, their leaving group ability in nucleophilic substitution reactions decrease in the order.
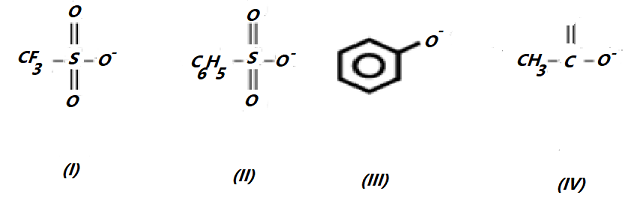
(A) $I > II > III > IV$
(B) $I > II > IV > III$
(C) $IV > I > II > III$
(D) $IV > III > II > I$
Answer
233.1k+ views
Hint: In this question we have given some structures of some compounds in which we have to find the decreasing order of their leaving group ability as in the form of nucleophilic substitution reaction. As this is attached to a $s{p^{3 - }}$ hybridised carbon so there will also be an effect from this.
Complete Step by Step Solution:
As we know that, when attached to a $s{p^{3 - }}$ hybridised carbon, their decreasing order of their leaving group ability in nucleophilic substitution reaction is $I > II > IV > III$. Because the reason for each position are given below:
In the structure no. (I) we get that, due to the presence of electron withdrawing effect present in the tri fluoro methyl group and there are 3 Oxygen atoms present with negative charge. From which (I) is the best leaving group.
(II) is a better leaving group than (IV) because (II) has a far greater degree of negative charge delocalization than (IV) does.
Due to the delocalization of the negative charge over the C atoms, III is the poorest leaving group.
Resonance has a stronger stabilising impact on an anion when the negative charge is delocalized over O atoms than when the negative charge is delocalized over C atoms.
Therefore, from taking all conclusions the correct answer is $I > II > IV > III$ .
Hence the correct option is (B).
Note: This question is topic under the reactions of haloalkanes. As for haloalkanes, there is a process by which haloalkanes go through an elimination process, which is, When sodium or potassium hydroxide is present, halogenoalkanes go through elimination processes.
Complete Step by Step Solution:
As we know that, when attached to a $s{p^{3 - }}$ hybridised carbon, their decreasing order of their leaving group ability in nucleophilic substitution reaction is $I > II > IV > III$. Because the reason for each position are given below:
In the structure no. (I) we get that, due to the presence of electron withdrawing effect present in the tri fluoro methyl group and there are 3 Oxygen atoms present with negative charge. From which (I) is the best leaving group.
(II) is a better leaving group than (IV) because (II) has a far greater degree of negative charge delocalization than (IV) does.
Due to the delocalization of the negative charge over the C atoms, III is the poorest leaving group.
Resonance has a stronger stabilising impact on an anion when the negative charge is delocalized over O atoms than when the negative charge is delocalized over C atoms.
Therefore, from taking all conclusions the correct answer is $I > II > IV > III$ .
Hence the correct option is (B).
Note: This question is topic under the reactions of haloalkanes. As for haloalkanes, there is a process by which haloalkanes go through an elimination process, which is, When sodium or potassium hydroxide is present, halogenoalkanes go through elimination processes.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




