
Complex formation method is used for the extraction of:
(A) $Zn$
(B) $Ag$
(C) $Hg$
(D) $Cu$
Answer
232.8k+ views
Hint: In complex formation method, an ore is converted into its pure form by the formation of a complex. The transition metals can be extracted through this method. The transition method can form complexes because they are small in size and have d-electrons to form new bonds. They can receive lone pairs of ligands and form complexes because of their small size, d-electrons, and high nuclear size.
Complete Step by Step Answer:
Out of the given options, only silver ($Ag$) can be extracted by a complex formation method. An ore of silver is converted into pure silver with the help of a complex formation method. An ore of silver ($Ag$) is treated with sodium cyanide ($NaCN$) by passing an air current through it and gives silver sodium cyanide ($Na[Ag{{(CN)}_{2}}]$). The silver sodium cyanide is then treated with zinc to form pure silver.
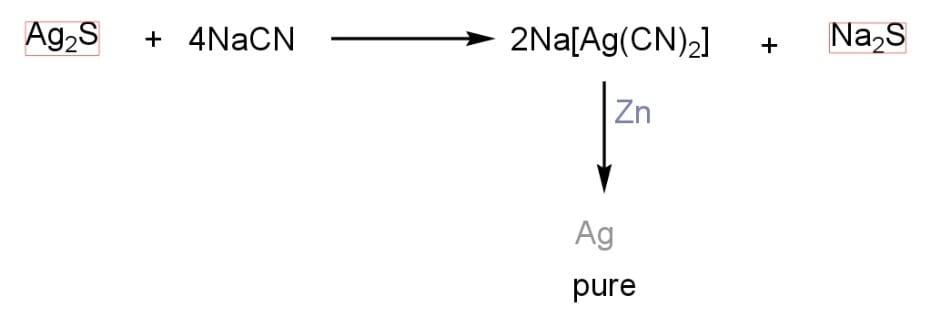
Correct Option: (B) $Ag$.
Additional Information: Metals exist in nature in their impure form, i.e., ore. The pure metal is then extracted from the ores. Different types of ores can be converted into their pure form by different methods of extraction. Metal extraction methods include chemical separation, froth floatation, calcination, roasting, magnetic separation, hydrolytic method, and others.
Note: The extraction of other elements given in the option is done by other methods. The extraction of zinc ($Zn$) and copper ($Cu$) is done by the electrolysis method. The extraction of mercury ($Hg$) is done by the distillation process.
Complete Step by Step Answer:
Out of the given options, only silver ($Ag$) can be extracted by a complex formation method. An ore of silver is converted into pure silver with the help of a complex formation method. An ore of silver ($Ag$) is treated with sodium cyanide ($NaCN$) by passing an air current through it and gives silver sodium cyanide ($Na[Ag{{(CN)}_{2}}]$). The silver sodium cyanide is then treated with zinc to form pure silver.

Correct Option: (B) $Ag$.
Additional Information: Metals exist in nature in their impure form, i.e., ore. The pure metal is then extracted from the ores. Different types of ores can be converted into their pure form by different methods of extraction. Metal extraction methods include chemical separation, froth floatation, calcination, roasting, magnetic separation, hydrolytic method, and others.
Note: The extraction of other elements given in the option is done by other methods. The extraction of zinc ($Zn$) and copper ($Cu$) is done by the electrolysis method. The extraction of mercury ($Hg$) is done by the distillation process.
Recently Updated Pages
Types of Solutions in Chemistry: Explained Simply

JEE General Topics in Chemistry Important Concepts and Tips

JEE Extractive Metallurgy Important Concepts and Tips for Exam Preparation

JEE Amino Acids and Peptides Important Concepts and Tips for Exam Preparation

JEE Atomic Structure and Chemical Bonding important Concepts and Tips

Electricity and Magnetism Explained: Key Concepts & Applications

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




