




What is an Oxidising Agent?
Chemistry deals with various types of reactions, and one of the most important types of reactions in Chemistry is redox reactions. Oxidising agents are used in redox reactions. The burning of different types of fuel, electrochemical processes used for the extraction of metals and non-metals, reactions occurring during battery operation, corrosion of metals, the manufacture of caustic soda, etc., are some examples of redox reactions. In a redox chemical process, an oxidising agent is a material that "accepts" or "receives" an electron from a reducing agent (also known as an oxidant, oxidiser, electron recipient, or electron acceptor). So every substance that oxidises another substance is an oxidant. The oxidation state of an oxidising agent decreases in a redox reaction.
Consider the following reaction:
$2Na(s)\longrightarrow 2Na^+(g) +2e^-$
$Cl_2(g)+2e^- \rightarrow 2Cl^-(g)$
$2Na(s)+Cl_2(g)\rightarrow 2NaCl(s)$
Here, 1 sodium atom loses 1 electron and forms sodium ion. Hence, it is undergoing an oxidation reaction. Similarly, 1 chlorine atom gains 1 electron to form chloride ion and hence, it is undergoing a reduction reaction. Thus, as per definition, chlorine is the acceptor of electrons and is the oxidising agent. On the other hand, sodium which is oxidised is the reducing agent.
Oxidising Agent Definition
We can define an oxidising agent in the following ways:
An Electron Acceptor: Oxidising agents are one of the reactants in a redox reaction whose atoms remove at least one electron from another atom. In other words, an oxidising agent gains at least one electron during such a reaction.
The general electron acceptors are oxygen, nitrate, ferrocenium ion, iron (III), manganese (IV), sulphate, carbon dioxide etc.
Consider the following reaction in which ammonia reacts with oxygen . Oxygen is the oxidising agent here:
$4NH_3+5O_2\rightarrow4NO+6H_2O$
Oxygen undergoes a reduction reaction as oxidation state changes from 0 to -2 and acts as electron acceptor and nitrogen undergoes an oxidation reaction.
An Atom-Transferring Substance: An oxidising agent can be a substance or species that gives away at least one electronegative atom to another chemical species in a redox reaction. The electronegative atom is generally an oxygen atom. Some examples of oxidising agents acting as atom transferring substances are MnO4- (permanganate), CrO42- (chromate), and ClO4- (perchlorate).
In the following reaction, oxalic acid is oxidised to carbon dioxide and the permanganate ion is reduced to the Mn2+ ion. Here, permanganate ion or MnO4- ion acts as an oxidising agent and oxalic acid as reducing agent.
$2MnO_4^-(aq)+5H_2C_2O_4(aq)+6H^+(aq)\longrightarrow 10CO_2(g) +2Mn^{2+}(aq)+8H_2O(l)$
Thus, we can summarise:
Oxidation: Loss of electron(s) by any substance.
Reduction: Gain of electron(s) by any substance.
Oxidising Agent: Accepts electron(s).
Reducing agent: Donates electron(s).
Factors Affecting the Power of Oxidising Agent
The oxidation number or oxidation state denotes the number of electrons in an atom which can be shared, lost or gained when it reacts or forms a chemical bond with another element. Oxidising agents generally are present in their highest possible oxidation state, and hence they undergo fast reduction reaction.
The main factors that affect are oxidising property are:
Ionisation Potential: The ionisation energy, ionisation potential, is the total amount of energy required to free an electron from the outermost shell of an atom in isolated gaseous state or also known as first ionisation potential. Oxidising agents usually have higher ionisation potential and hence these do not lose electrons easily and they function as electron acceptors.
Electronegativity: It is the measure of how much an atom can attract a bonded electron compared to another atom in a compound. The oxidising agents have higher electronegativity as they have a tendency to attract electrons strongly. The electronegativities increase as we move left to right in a periodic table. The strongest elemental oxidising agent is fluorine, and it is the most electronegative element in the periodic table. Diatomic oxygen (O2), chlorine (Cl2), and ozone (O3) are some examples of elemental oxidising agents. Other compounds which have good oxidising agents are permanganate, chromate ions, as well as nitric acid, nitric acid etc. This is because they become more electronegative as their atoms have large oxidation state.
Atomic Size: As we move left to right in the periodic table, the atomic size decreases. This is because here the number of shells are the same, but the nuclear charge increases. The higher nuclear charge causes pulling of electrons from the outermost shell towards the nucleus, thereby decreasing the size. The smaller atomic size molecules like fluorine, oxygen, chlorine show good oxidising properties.
Metallic or Non-Metallic Character: Non-metals tend to gain electrons in chemical reactions and have a high attraction for electrons. Hence, some of them act as good oxidising agents. The element fluorine is the most reactive non-metal and is thus a good oxidising agent.
Number of Valence Electrons: The elements with more valence electrons like fluorine, oxygen, chlorine etc. are good oxidising agents as they tend to gain electrons.
The potential of the half-reaction or half-cell measured against the standard hydrogen electrode under standard conditions is called the standard electrode potential for that half-cell. The standard electrode potential occurs in an electrochemical cell with temperature = 298K, pressure = 1atm and concentration = 1M. The standard electrode potential of a cell is given by the symbol \[{{E}^{\circ}}\] cell. The compounds with higher standard reduction potential have high oxidising power, and the compounds with low standard reduction potentials are good reducing agents. Below is a list of some oxidising agents arranged on the basis of standard reduction potentials.
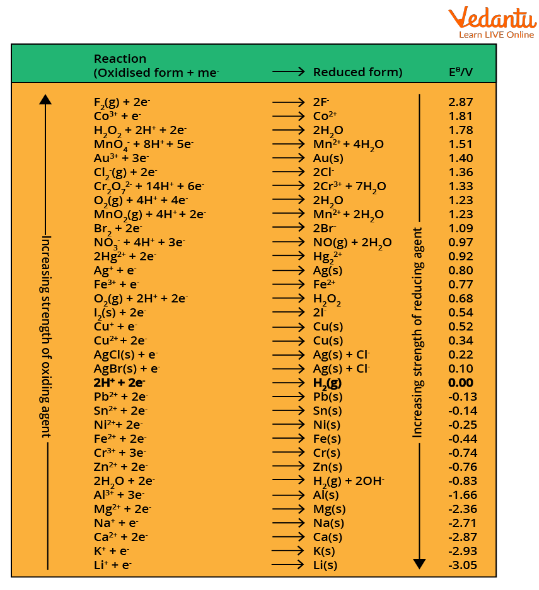
The Standard Reduction Potentials
Some Examples of Oxidising Agents
Oxygen compounds like Oxygen (O2) and Ozone (O3)
Nitric acid (HNO3) and nitrate compounds
Permanganate compounds such as potassium permanganate (KMnO4)
Halogens such as fluorine (F2), chlorine (Cl2)
Hypochlorite, chlorite, chlorate, perchlorate, for example: household bleach (NaClO)
Hydrogen peroxide (H2O2) and other inorganic peroxides
Sodium dichromate
Nitrogen compounds, such as nitrous oxide, nitrogen dioxide/dinitrogen tetroxide (NO2 / N2O4)
Potassium nitrate (KNO3)
Applications of Oxidising Agents
There are many commercial and industrial applications of oxidising agents such as:
Oxidising agents such as chlorine and ozone are used in the disinfection of water during water purification.
The clothes or fabrics are bleached using oxidising agents such as sodium hypochlorite, sodium chlorite, or sulphuric acid.
The three different solutions of oxidising agents commonly used in daily life are hydrogen peroxide (cleaning agent, disinfectant, etc.), liquid bleach (sodium hypochlorite-NaClO), chlorine compounds like calcium hypochlorite (65% available chlorine, disinfectant in swimming pools).
The biological processes such as metabolism and photosynthesis also require the presence of oxidising agents.
Redox reactions are used in industrial processes as well. For example, the major source of energy in industries are combustion reactions, which is also a type of redox reaction.
Conclusion
An oxidising agent is a substance which is present in a redox (oxidation-reduction) reaction, and gains electrons from other substances. These reduce themselves in order to oxidise other substances. Some oxidants lose oxygen or gain hydrogen in order to oxidise other substances. Fluorine is the strongest elemental oxidising agent. In redox reactions, the oxidising agent undergoes a reduction reaction. Many chemical reactions in industries and day to day life are redox reactions.
Oxidising agents also have many applications. They are used in combustion reactions, photosynthesis, metabolism, disinfection, bleaching, etc. Commonly used oxidisers are chlorine, oxygen, ozone, sodium hypochlorite, sulphuric acid etc.
FAQs on Oxidising Agent - Important Concepts with Applications and Examples for JEE
1. What are antioxidants?
Antioxidants are substances that prevent oxidation or a chemical process that can result in free radicals and cascade events that could harm an organism's cells. These processes may be inhibited by antioxidants like thiols or ascorbic acid (vitamin C). Plants and mammals maintain complex networks of overlapping antioxidants, including glutathione, to manage oxidative stress.
Vitamins A, C, and E are the only dietary antioxidants. In addition, industrial chemicals employed antioxidants to preserve food and cosmetics and to stop oxidation in synthetic rubber, plastics, and fuels.
2. What is the role of oxidising agents in water purification?
The oxidising agents such as chlorine and ozone are used in the disinfection of water in the purification process. Disinfection means killing the pathogenic bacteria that causes various diseases. Chlorine has been used for more than a century as a primary water disinfectant. Chlorine is a hazardous chemical and needs to be handled carefully and safely.
Disinfection systems using chlorine gas also have the disadvantage of producing toxic disinfection by-products. Ozone oxidises organic material in the membranes of bacteria, viruses, and parasites which weakens and kills these pathogens. Ozone also oxidises iron, manganese and copper which can be then easily filtered from water.
























