




Where do Metals Occur?
Elements are the building blocks of all matter present in the universe. How do these elements occur in the earth's crust, atmosphere, sea and how are they obtained? Metals account for 80 percent of all known elements, and our primary focus on this topic will be on metal extraction.
Elements are found in nature either in their free state (native state) or in their combined state, i.e. as compounds. In their natural state, some elements are not easily attacked by moisture, oxygen or carbon dioxide from the air. For example, carbon, gold, platinum and so on. Elements that are easily attacked by moisture, oxygen and carbon dioxide in the air exist in the combined state as minerals, i.e. oxides (e.g.,Fe2O3, Al2O3, MnO2). Furthermore, the majority of metal halides that are soluble in water are found in lakes and seas, whereas the majority of metal oxides and sulphides that are insoluble are found in rock.
Occurrence of Metals
The majority of metals are found in the earth's crust in the form of combined compounds known as minerals. Thus, minerals are naturally occurring chemical substances in the form of which metals occur in the earth along with impurities.
However, the relative abundance of these metals in the earth's crust varies from one metal to the next. Aluminium, for example, is the third most abundant element in the earth's crust by mass (approx. 8.3 %), after oxygen (46.6 %) and silicon (27.7 %). Iron is the second most abundant metal in the earth's crust (about 4.7 % by mass). Iron is also a necessary component of biological systems. Metals can be found in a variety of minerals, but metal extraction is difficult due to either low concentration or the presence of impurities.
Ore
An ore is a mineral from which the metal can be extracted easily and cheaply. All ores are minerals, however, all minerals are not necessary to be ores. Aluminium, for example, is found in the earth's crust as two minerals: bauxite and china clay. Aluminium can be extracted from bauxite simply and cost-effectively, whereas no simple and cost-effective method exists for extracting aluminium from clay. As a result, bauxite is the ore of aluminium.
Extraction of Metal
Let's study the process of extraction of metals from their ores (i.e. metallurgy). The process used to extract a metal is determined by its physical and chemical properties, as well as the impurities associated with it. Since different metals have different physical and chemical properties, they require different extraction methods. There are some procedures and processes for metal extraction. Let's look at some of the methods for ore concentration, which is the first step in metal extraction.
Ores extracted from the earth's crust are never pure. They are usually associated with earthy and siliceous impurities (along with other minerals' impurities) known as gangue or matrix. Before the metal can be extracted, these impurities must be removed from the ore.
1. Electromagnetic Separation
When the ore or the impurities associated with it are magnetic in nature, this method of concentration is used. The powdered ore is dropped over a conveyor belt, moving around two rollers, one of which contains an electromagnet. The magnetic roller attracts the magnetic particles as the ore particles roll over the belt. As a result, two heaps form independently. The magnetic particles are collected in the heap formed beneath the magnetic roller, while the non-magnetic impurities are collected in the heap formed away from the magnetic roller. In the case of tinstone, the magnetic tungstates form a heap beneath the magnetic roller, while the ore particles, i.e., SnO, forms a separate heap away from the magnetic roller.
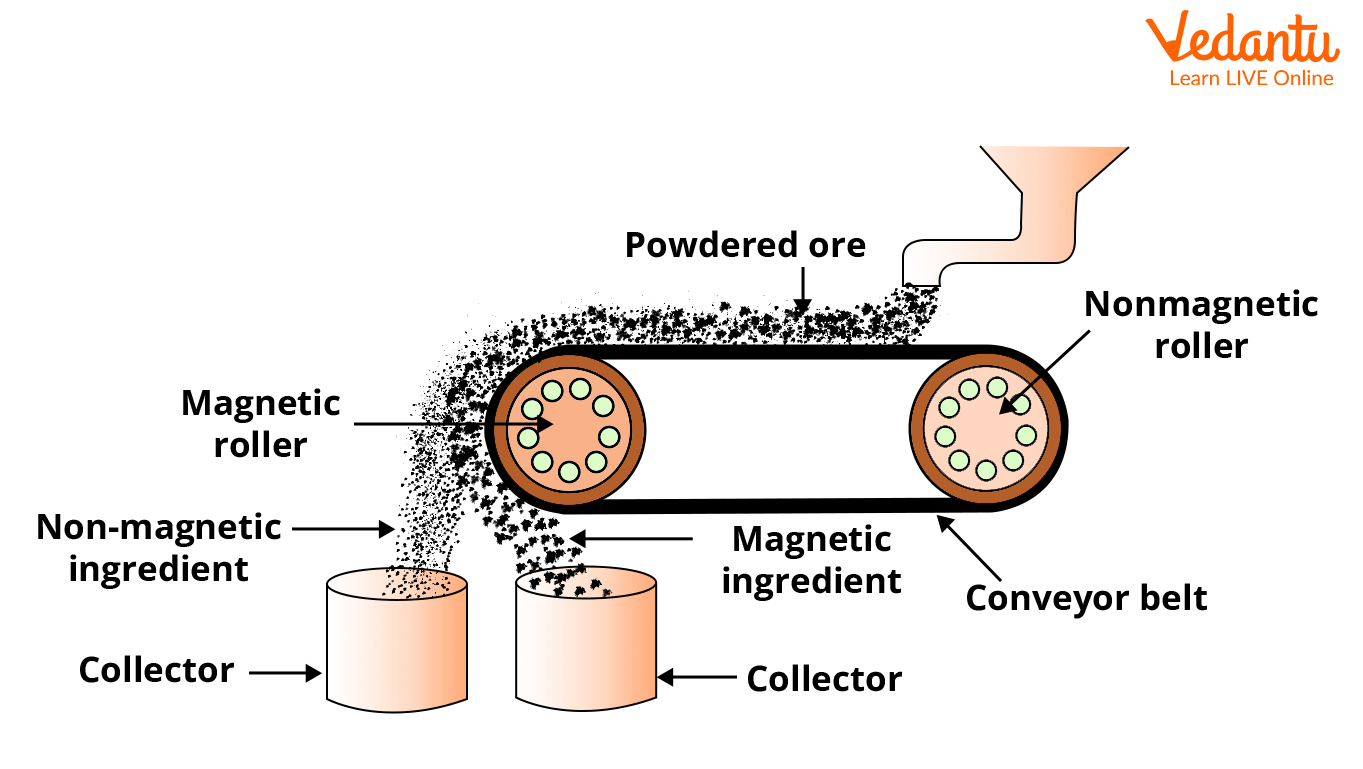
Electromagnetic Separation Method
2. Hydraulic Washing
Also called the levigation method, it is used to purify heavier ores such as oxides, carbonates, native gold etc. The ore is washed with running water, where the lighter impurities are washed away, whereas heavier particles settle down.
3. Froth Flotation Method
This method is commonly used to concentrate sulphide ores such as zinc blende (ZnS), copper pyrites (CuFeS2) and others. The surface of sulphide ores is preferentially wetted by oils, whereas the surface of gangue is preferentially wetted by water.
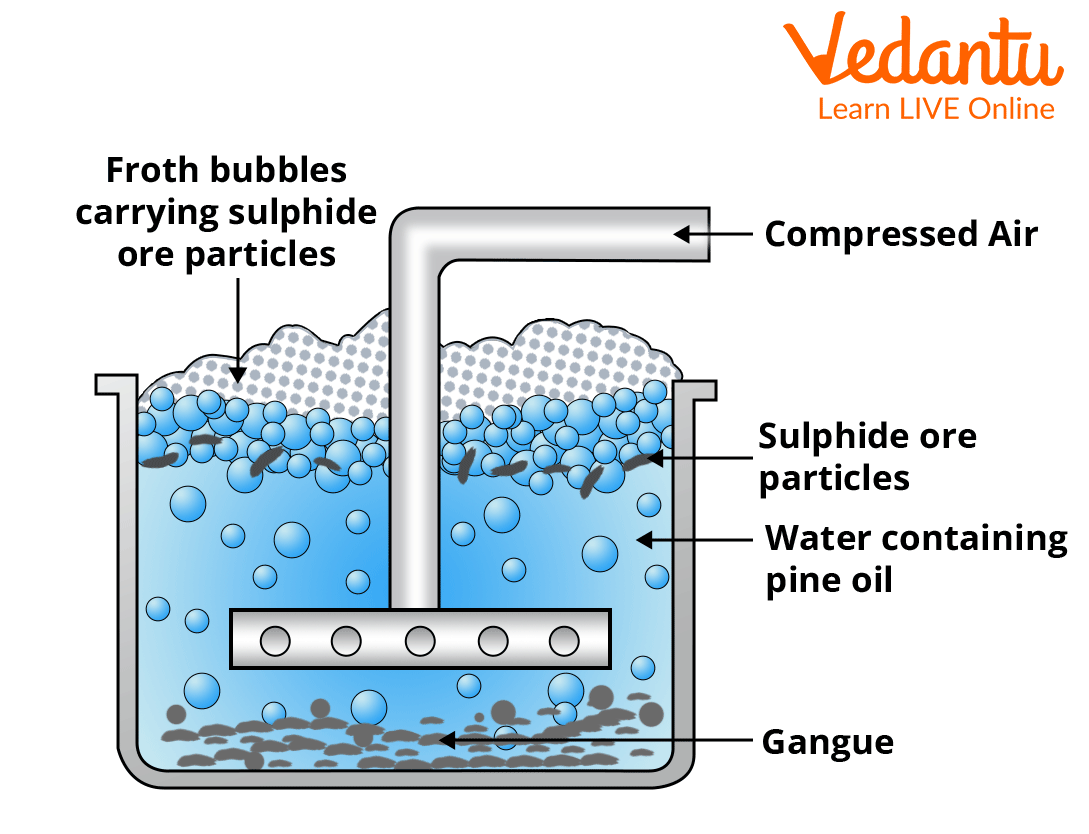
Froth Flotation Method
In a tank, the ore is crushed into a fine powder and mixed with water to form a suspension. Collectors (e.g pine oil, xanthates) are added to this suspension to improve the non-wettability of the ore particles. Froth-stabilisers (e.g., cresols and aniline) are added to stabilise the froth.
The rotating paddle violently agitates the suspension, drawing in air and causing frothing. During this process, the ore particles that are preferentially wetted by the oil become lighter and rise to the surface with the froth, whereas the gangue particles that are preferentially wetted by water become heavier and settle at the tank's bottom. The froth is removed. It is allowed to collapse before being dried, to obtain the concentrated ore.
4. Leaching
Leaching is the process of extracting the required metal from an ore in the form of an aqueous solution of its selected compound, by treating the ore with acid, base or another reagent. For example, bauxite ore is continuously stirred with 45 percent NaOH at 200-250 °C, to collect the solution of sodium aluminate Na(Al(OH)4). The sodium aluminate is neutralised by passing carbon dioxide gas and as a result hydrated Al2O3 is precipitated. The solution is seeded with freshly prepared samples of hydrated Al2O3, at this stage, which induces the precipitation. The solution is then filtered, the hydrated Al2O3 is then dried and heated at 1470 K temperature to give pure Al2O3.
5. Conversion of Concentrated Ore into Metal Oxide
Calcination: This method converts carbonate and hydrated oxide ores to their respective oxides, by heating them to temperatures well below their melting points, either in the absence or with a limited supply of air. Nonmetallic impurities such as S, P, As and others are removed as volatile oxides.
Roasting: By intensely heating sulphide ores, this process converts them to their respective oxides.
Conclusion
The Earth's crust is a repository for metals such as sulphur and silicon. Some of the major metals, such as chloride, are also reported in seawater. They can be found in both free and combined states. Metallurgy is the sum of all processes used to extract metals from their ores. Ores are minerals from which one or more metals can be extracted easily and profitably. As previously discussed, there are various methods for ore concentration. These include hydraulic, froth flotation, electromagnetic leaching, and other extraction methods.
FAQs on Occurrence of Metals and Their Extraction Process for JEE
1. Name and describe methods of extraction of sulphide ores and also the role of depressant in that method?
The froth flotation process is employed to purify sulphide ores. The depressant's role in the froth floatation process is to prevent one type of sulphide ore particle from forming the froth with air bubbles. To separate lead sulphide (PbS) ore from zinc sulphide (ZnS), for example, NaCN is used as a depressant. The reason for this is that NaCN forms a zinc complex $\mathrm{Na}\left[\operatorname{Zn}(\mathrm{CN})_{4}\right]$ on the surface of ZnS, which prevents it from forming a froth. Only PbS forms froth under these conditions and can thus be separated from ZnS ore.
$4 \mathrm{NaCN}+\mathrm{ZnS} \rightarrow \mathrm{Na}_{2}\left[\mathrm{Zn}(\mathrm{CN})_{4}\right]+\mathrm{Na}_{2} \mathrm{~S}$
2. Distinguish between "minerals" and "ores."
Minerals are naturally occurring chemical substances in the form of which metals and impurities occur on the earth. An ore is a mineral from which metal can be extracted easily and economically. From this we can say that all ores are minerals, all minerals are not necessary to be ores. Iron, for example, exists in the earth's crust as oxides, carbonates and sulphides. The oxides of iron extracted from these iron minerals are used to extract the metal. As a result, iron oxides are iron ores.

































