




How is the Naming of Alkenes, Alkynes and Alkenes Done?
We will discuss IUPAC naming of hydrocarbons here. Alkanes are the simplest hydrocarbons we know of. They have the general formula of CnH2n+2. They belong to the saturated hydrocarbon family. They contain only sigma bonds between carbon and hydrogen. Organic compounds form a homologous series in which successive compounds contain the same functional groups, and the "-CH2" groups differ from each other.
On the other hand, alkenes and alkynes are unsaturated hydrocarbons. Double bonds are seen in alkenes, and triple bonds are seen in alkynes. The longest hydrocarbon chain is selected and is called the parent chain in the case of alkanes. For alkenes and alkynes, hydrocarbon chains with double and triple bonds are selected as the parent chain. The parent chain is named using the Greek alphabet such as hepta and octa. The suffix "-ane" is used for alkanes, the suffix "-ene" is used for alkenes, and the suffix "yne" is used for alkynes.
For example, C2H6 is known as ethane, C2H4 is known as ethylene, and C2H2 is known as ethyne. The earliest we need to reach is a double or triple bond carbon atom. The positions of carbon atoms containing double bonds are indicated by numbers. For example, CH3CH = CHCH2CH3 is called Pent-2-en. If the carbon chain has multiple double bonds, use prefixes such as di, tri, etc., to indicate their number.
Rules - Nomenclature of Alkanes
Find and name the longest continuous carbon chain in the given hydrocarbon.
Identify and name the group associated with this chain.
Number the strands in order from the end closest to the substituent.
Identify the location of each substituent by the corresponding number and name.
Create a name for and list the groups alphabetically.
The prefixes di, tri, tetra, etc., indicate several groups of the same type.
For example:
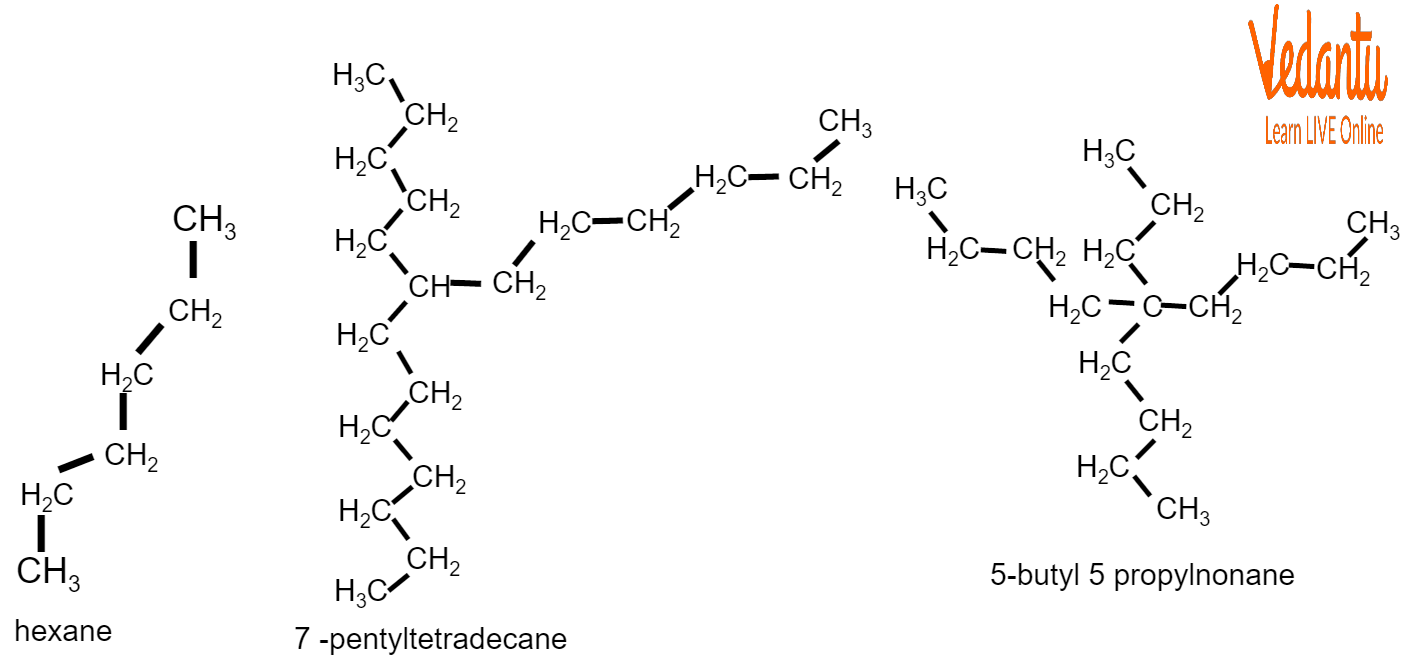
Examples of Nomenclature of Alkanes
Rules - Nomenclature of Alkenes
As per alkene naming rules, the longest carbon chain containing a carbon-carbon double bond is selected as the parent alkene.
The alkane suffix "an" is replaced with "en".
If double bonds occur two or three times in the main-chain, the alkenes are called dienes or trienes, respectively.
Double bond or side-chain position.
It is indicated by numbers 1, 2, 3, and so on.
The longest chain is numbered from the end, giving the lowest number to the carbon atom of the double bond, and is written just before the suffix "en".
When numbering chains, if the double bond gets the same number from both sides, the carbon chain is numbered so that the substituent gets the lowest number.
If you have more than one double bond, you must follow the rule of minimum sum.
The names and positions of other groups (substituents) are prefixed.
Another name of alkene is olefins.
For example:
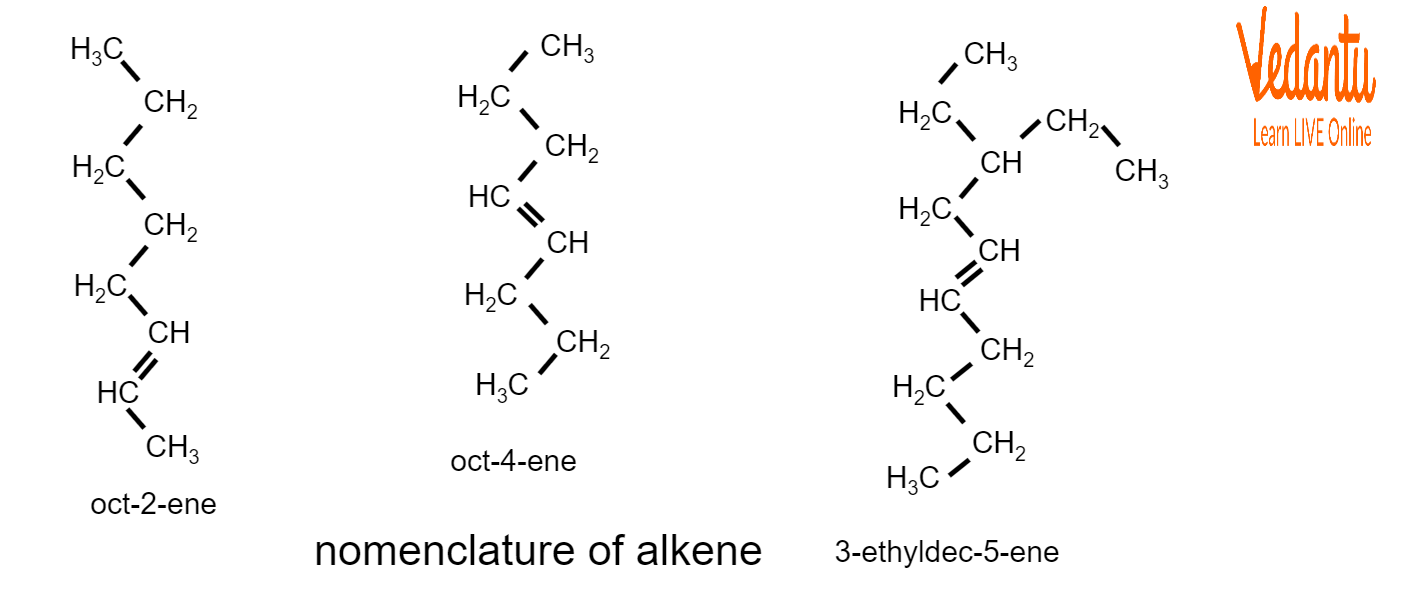
Examples of Nomenclature of Alkenes
Rules- Nomenclature of Alkynes
Find the longest carbon chain containing both carbons in a triple bond.
Number the longest strand starting from the end closest to the triple bond. 1-Alkynes are called terminal alkynes, and alkynes in other locations are called internal alkynes.
After numbering the chain with the longest and lowest number assigned to the alkyne, label each substituent with the corresponding carbon. Arrange the substituents in alphabetical order, writing the name of the molecule.
If you have more than one of the same substituents, use the prefixes di, tri, and tetra for the two, three, and four substituents, respectively.
These prefixes are not considered in alphabetical order.
If the molecule contains alcohol, follow the same rules and number the longest chain from the end closest to it.
However, the suffix is -ynol because the alcohol group takes precedence over the triple bond.
If there are two triple bonds in the molecule, find the longest carbon chain containing both triple bonds.
Number the longest strands, starting with the end closest to the triple bond that appears first.
Chains can be numbered from the end closest to the functional group that appears first.
For example:
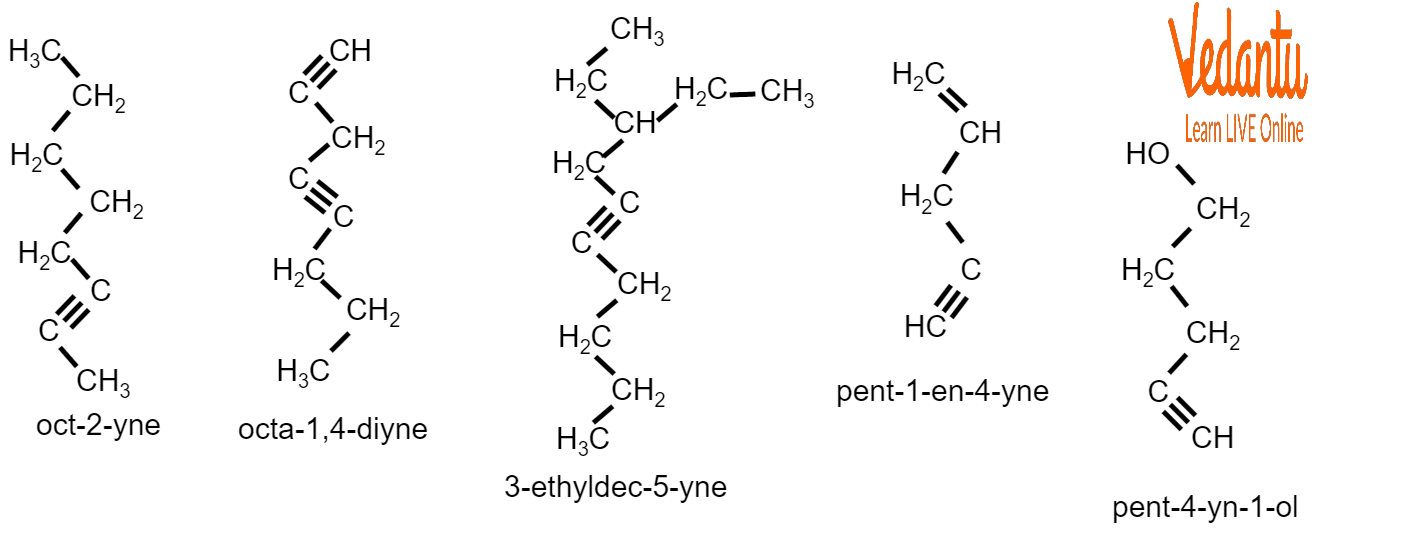
Examples of Nomenclature of Alkynes
Alkane Alkene Alkyne Chart
The chart below shows the naming of alkanes, alkenes, and alkynes having a particular number of carbon atoms in the parent chain.
Alkane Alkene Alkyne Chart
Saturated Hydrocarbons
Saturated hydrocarbons are hydrocarbons in which all carbon-carbon bonds are single bonds.
Hydrocarbons are the only organic compounds that contain only carbon and hydrogen.
As the name implies, saturated hydrocarbons are hydrocarbons that are "saturated" with all carbon atoms bonded to the other four atoms.
That is, there are no carbon-carbon multiple bonds in these organic compounds.
In general, "saturated hydrocarbon" refers to alkanes or acyclic hydrocarbons that contain only sp3 mixed carbon atoms. The general formula for alkanes is CnH2n+2.
Unsaturated Hydrocarbons
Unsaturated hydrocarbons are hydrocarbons in which one or more carbon atoms in a chain are bonded by double or triple bonds.
Alkenes and alkynes are unsaturated hydrocarbons.
Hydrocarbon alkenes have double bonds between carbon atoms.
Like alkenes, their molecular formulas increase by a certain amount as each carbon atom is added to the chain.
The family of compounds growing in this way is called the homologous series.
The general formula for alkenes is C2H2n. Note that this is the same as the alkane equation, except that two hydrogens are subtracted to account for the double bond.
Summary
We discussed IUPAC naming alkenes Alkanes, alkenes, and alkynes are simple hydrocarbon chains with no functional groups. The simplest organic compound is alkane. Alkanes have a single bond between carbon atoms and are called saturated hydrocarbons. Alkenes have at least one carbon-carbon double bond. Alkynes have one or more carbon-carbon triple bonds. Alkenes and alkynes are called unsaturated hydrocarbons.
The alkane, alkene and alkyne formulae are CnH2n+2 CnH2n and CnH2n-2 respectively. The rules for naming these hydrocarbons are almost the same. However, in the case of alkene and alkynes, rules and priority order due to double and triple bonds are additional.
FAQs on Naming of Alkenes, Alkynes and Alkanes - JEE Important Topic
1. Write the uses of saturated hydrocarbons.
The uses of saturated hydrocarbons are as follows:
The simplest alkane, methane, is used as fuel in some cars, water heaters, and furnaces.
In highly refined form, liquid methane also functions as rocket fuel.
Some cryogenic freezing systems use ethane as the refrigerant. It is also used in the production of ethylene.
The propellant in some aerosol sprays is a saturated hydrocarbon known as propane.
Octane is a vital component of gasoline to prevent engine damage.
Cycloalkanes are also used in motor fuels, diesel, petroleum gas, and other heavy oils.
Cycloalkane is also used in the production of rubber and nylon.
2. Write the uses of unsaturated hydrocarbons.
The uses of unsaturated hydrocarbons are as follows:
Many fruits can be artificially aged using alkenes; the other name of alkene is olefin. Mustard gas, a toxic gas used in chemical weapons, can be manufactured using alkenes.
Unsaturated hydrocarbons are very useful organic compounds in the production of plastics.
LDPE, a variant of low-density polyethylene, is used to make grocery bags.
Polystyrene is used to manufacture egg packs, disposable cups, and other useful products.
Industrial chemicals, such as alcohol, use alkenes in manufacturing.
Some unsaturated hydrocarbons are used as general anaesthetics.
























