




Stepwise Mechanism of Kolbe Electrolysis for Alkane Preparation
The Kolbe's Electrolytic Method is a key topic in JEE Main Organic Chemistry, known for the preparation of symmetrical alkanes by electrolyzing aqueous solutions of sodium or potassium salts of carboxylic acids. This process, often called Kolbe electrolysis, demonstrates how hydrocarbons can be generated through a radical mechanism, making it essential both for concept clarity and for solving reaction-based numericals.
Kolbe's Electrolytic Method: Principle and Importance
Developed by Hermann Kolbe, this method involves the electrolytic decarboxylation of carboxylate ions at the anode, generating free alkyl radicals which couple to produce alkanes. Kolbe’s electrolytic method is primarily used to synthesize alkanes with an even number of carbon atoms, making it different from some other hydrocarbon preparation techniques. The reliance on electrochemical principles also helps highlight an important link between electrolysis and organic synthesis for JEE aspirants.
General Equation of Kolbe's Electrolysis
The typical reaction in Kolbe’s electrolytic method can be summarised as:
| Step | Reaction Equation |
|---|---|
| At Anode (Oxidation) | 2 RCOO– → 2 R· + 2 CO2 + 2 e– |
| Radical Combination | 2 R· → R–R (Alkane) |
| At Cathode (Reduction) | 2 H2O + 2 e– → H2 + 2 OH– |
Here, RCOO– represents the carboxylate ion derived from the corresponding acid salt (e.g. sodium acetate). The main product is a higher alkane (R–R), and CO2 and H2 are side-products. Note that only alkanes with an even number of carbons can be prepared directly.
Detailed Mechanism of Kolbe Electrolysis
The Kolbe reaction mechanism proceeds through the following steps at the electrodes in the electrolytic cell:
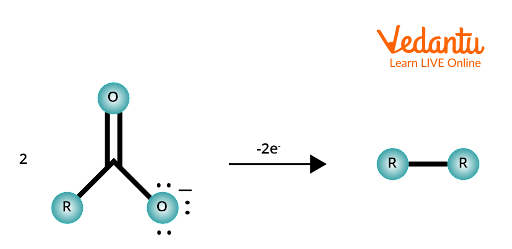
- At the anode, the carboxylate ion (RCOO–) loses an electron (oxidation) forming a carboxyl radical (RCOO·).
- The carboxyl radical rapidly decomposes, losing carbon dioxide (CO2) to form an alkyl radical (R·).
- Two alkyl radicals combine to yield an alkane (R–R), the chief organic product.
- Meanwhile, at the cathode, water is reduced to hydrogen gas and hydroxide ions.
For example, using sodium acetate (CH3COONa): at the anode, 2 CH3COO– → 2 CH3· + 2 CO2 + 2 e–. The methyl radicals (CH3·) then couple: 2 CH3· → C2H6 (ethane).
Key Features and Applications
Kolbe electrolysis is frequently asked in JEE Main as an example of electrolytic decarboxylation and radical coupling. It's best applied for:
- Preparation of symmetrical alkanes from pure carboxylic acid salts.
- Illustrating the use of electrolytic redox reactions in organic chemistry.
- Reinforcing radical mechanisms relevant to organic compound transformations.
- Comparative study with other alkane preparation methods.
A typical exam application: If sodium propionate (CH3CH2COONa) is electrolyzed, the chief product is n-butane (C4H10), demonstrating that the resulting alkane has double the number of carbons as the starting acid substituent (excluding the carboxyl carbon).
Limitations and Comparison with Other Methods
Some practical limitations exist for Kolbe’s electrolytic method, important for MCQs and reasoning questions:
- Only symmetrical alkanes can be formed; methane cannot be obtained, since methyl radicals (·CH3) cannot pair alone to make CH4.
- A mixture of two different sodium salts gives a mixture of alkanes (cross-coupling), resulting in impure products.
- Works best for aliphatic carboxylic acids; aromatic acids and tertiary acids fail or yield poor results.
- Competing side reactions (e.g. oxygen evolution at anode) can reduce alkane yield.
| Kolbe’s Method | Wurtz Reaction |
|---|---|
| Uses carboxylate salts; electrolysis needed | Uses alkyl halides and sodium; heat in dry ether |
| Even-numbered, symmetrical alkanes only | Mixture of alkanes possible |
| No methane formed | Can form methane (with methyl halide) |
Popular Variants and Advanced Tips
Remember these exam-focused points for Kolbe's electrolytic method:
- Graphite electrodes are typically used for high stability during electrolysis.
- The method is often called "Kolbe synthesis" when referring to salicylic acid preparation (with sodium phenoxide and CO2), but the synthesis of alkanes is the classic Kolbe electrolysis reaction.
- For maximum yield, use pure, concentrated aqueous salt solutions and control the applied voltage.
- In exam numericals, identify the radical and double its carbon count for the expected alkane.
Solved Example: JEE Main-Style Problem
Question: Electrolyzing a solution of sodium butyrate (CH3CH2CH2COONa) gives?
(A) n-Octane (B) Butane (C) n-Hexane (D) Ethane.
Solution: The butyrate ion forms CH3CH2CH2· radicals. Coupling: 2 × C4 → C8 product → n-Octane (Option A).
Why Kolbe’s Electrolytic Method is Vital for JEE Main
JEE Main often tests Kolbe’s electrolytic method for its classic mechanism, radical pairs, and comparison with methods like Wurtz reaction and soda lime decarboxylation. Pay close attention to the structure of starting acids, electrode reactions, and possible side-products, as well as application to mixed-salt problems. Make sure to revise redox concepts, electrochemical cells, and organic radicals for comprehensive understanding.
For more practice and conceptual clarity on Kolbe's electrolytic method and related reactions, review Vedantu’s JEE Main Chemistry resources and previous year question sets.
FAQs on Kolbe’s Electrolytic Method Explained with Examples
1. What is Kolbe's electrolytic method?
Kolbe's electrolytic method is a classic technique for preparing alkanes by electrolyzing sodium or potassium salts of carboxylic acids in aqueous solution.
Key steps of the process include:
- At the anode, decarboxylation occurs, producing alkyl radicals.
- These radicals quickly combine to form symmetrical alkanes.
- Hydrogen gas is typically liberated at the cathode.
2. How does Kolbe’s electrolytic method prepare alkanes?
Kolbe's electrolytic method prepares alkanes by the electrolysis of the sodium or potassium salts of carboxylic acids.
Key steps are:
- Electrolysis setup with aqueous carboxylate solution.
- At the anode, decarboxylation removes CO2, generating alkyl radicals.
- Two alkyl radicals join to form a higher alkane (usually of even carbon number).
- Example: Electrolysis of sodium acetate gives ethane as the major product.
3. What is the equation for the Kolbe's electrolysis reaction?
The general Kolbe electrolysis equation involves the reaction of two carboxylate ions at the anode.
General equation:
2 RCOO- → 2 R• + 2 CO2 + 2 e-
2 R• → R–R (alkane)
where RCOO- is the sodium or potassium salt of a carboxylic acid and R–R is the resulting alkane. This explanation is crucial for board exams and competitive chemistry tests.
4. Which products are formed in Kolbe electrolysis?
Kolbe electrolysis primarily forms symmetrical alkanes of even carbon numbers, but also produces side products:
- Main product: Alkane, where two identical alkyl groups combine (e.g., ethane from sodium acetate).
- Byproducts: Carbon dioxide (CO2) at the anode and hydrogen gas (H2) at the cathode.
- Possible side products: If a mixture of two carboxylates is used, cross-coupled alkanes are possible. Side reactions can also yield alkenes or alcohols in minor amounts.
5. What is Kolbe's electrolytic method for preparation of alkanes?
Kolbe's electrolytic method is used to prepare symmetrical alkanes by electrolyzing sodium or potassium salts of carboxylic acids.
Steps include:
- Electrolyzing the salt solution in water using graphite electrodes.
- Decarboxylation at the anode creates alkyl radicals which couple to form alkanes.
- Hydrogen is released at the cathode.
6. What happens at the anode and cathode in Kolbe’s method?
In Kolbe's electrolytic method, different reactions occur at each electrode:
- Anode (oxidation): Carboxylate ions release electrons, lose CO2, and form alkyl radicals. These combine to give alkanes.
- Cathode (reduction): Water gains electrons, producing hydrogen gas and hydroxide ions.
- Anode: RCOO- → R• + CO2 + e-
- Cathode: 2 H2O + 2 e- → H2 + 2 OH-
7. Can Kolbe’s method be used to prepare methane?
No, Kolbe's electrolysis cannot be used to prepare methane, because two methyl radicals (CH3•) would need to combine. However, their generation is not feasible under these conditions due to instability and side reactions, so ethane or higher alkanes are typically produced instead.
- Preparation of methane requires alternative methods.
- This is a common exam question in JEE and CBSE chemistry.
8. What is Kolbe's synthesis and how is it different from Kolbe's electrolytic method?
Kolbe's synthesis usually refers to the preparation of salicylic acid (aromatic hydroxy acids) from sodium phenoxide and carbon dioxide, not to alkane preparation. Kolbe's electrolytic method, meanwhile, is used to make alkanes via electrolysis of carboxylate salts.
- Kolbe's synthesis: Creates substituted aromatic acids.
- Kolbe's electrolytic method: Produces symmetrical alkanes via decarboxylation and dimerization of alkyl radicals.
9. What are the limitations of Kolbe’s electrolytic method?
The Kolbe electrolysis method has several important limitations:
- Only suitable for the preparation of alkanes with even numbers of carbon atoms.
- Cannot produce methane.
- If a mixture of two different carboxylic acid salts is used, a variety of alkanes may form (mixture issue).
- Formation of side products such as alkenes, alcohols, or gases is possible.
- Works best with aliphatic (not aromatic) acids.
10. How does Kolbe’s electrolytic method differ from the Wurtz reaction for preparation of alkanes?
Kolbe's electrolytic method and the Wurtz reaction both make alkanes, but they use different reactants and mechanisms:
- Kolbe's method: Electrolysis of carboxylate salts; forms alkane by coupling of alkyl radicals.
- Wurtz reaction: Reacts alkyl halides with sodium metal in dry ether.
- Kolbe: Best for symmetrical alkanes of even carbon number.
- Wurtz: Suited for both symmetrical and some unsymmetrical alkanes.
11. Why is graphite used as an electrode in Kolbe's electrolytic method?
Graphite is commonly used as the anode in Kolbe’s electrolysis because:
- It is chemically inert, preventing contamination of the product.
- It withstands the oxidation conditions during electrolysis.
- Graphite provides good electrical conductivity for efficient radical generation.
12. What happens if a mixture of two carboxylic acid salts is used in Kolbe’s method?
Using a mixture of two different carboxylate salts in Kolbe’s electrolysis can produce:
- Three different alkanes: two symmetrical (from identical radicals) and one unsymmetrical alkane (from cross-coupling).
- The resulting product mixture complicates product isolation and yield.























