
An element crystallizes in bcc structure. The edge length of its unit cell is 288 pm. If the density of the crystal is 7.2 g \[cm ^{- 3}\]. what is the atomic mass \[\;\left( {in{\text{ }}g/mol} \right)\] of the element?
A. 51.8
B. 103.6
C. 25.9
D. 207.2
Answer
242.4k+ views
Hint: Body-centered cubic unit cell is one kind of cubic lattice. In this cubic lattice each unit cell contains one atom at its body center. And contains one atom at the eight corners of the unit cell.
Formula used: Density = \[\dfrac{{(M \times z)}}{{(N \times {a^3})}}\]= \[\dfrac{{(molecular{\text{ }}weight){\text{ (}}number{\text{ }}of{\text{ }}atoms{\text{ }}per{\text{ }}cell){\text{ }}}}{{{{(edge{\text{ }}length)}^3}{\text{ (}}Avogadro{\text{ }}number)}}\]
Complete step by step answer:
The structure is shown below,
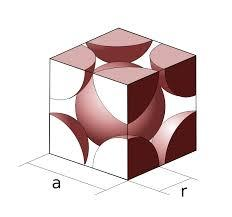
We know that,
Density = (mass)/(volume)
Applying this concept for calculating the density of the unite cell, we get,
Density = \[\dfrac{{(M \times z)}}{{(N \times {a^3})}}\]
= \[\dfrac{{(molecular{\text{ }}weight){\text{ (}}number{\text{ }}of{\text{ }}atoms{\text{ }}per{\text{ }}cell){\text{ }}}}{{{{(edge{\text{ }}length)}^3}{\text{ (}}Avogadro{\text{ }}number)}}\]
= \[7.2 = \dfrac{{M \times 2}}{{{{(288 \times {{10}^{10}})}^3} \times 6.022 \times {{10}^{23}}}}\]
Hence,
\[
7.2 = \dfrac{{M \times 2}}{{{{(288 \times {{10}^{10}})}^3} \times 6.022 \times {{10}^{23}}}} \\
M = \dfrac{{7.2 \times {{(288 \times {{10}^{10}})}^3} \times 6.022 \times {{10}^{23}}}}{2} \\
M = 51.77 \\
\]
Therefore, the molecular weight of the element is \[51.77gm/mol\].
Additional information:
In the B.C.C the closest distance between two atoms in the unit lattice is\[\dfrac{{\sqrt {\text{3}} a}}{2}\]. Where a is the edge length of the unit lattice.
Now according to this formula \[\dfrac{{\sqrt {\text{3}} a}}{{\text{2}}}{\text{ = 173pm}}\]
Therefore, the edge length is,
\[
\dfrac{{\sqrt {{\text{3a}}} }}{{\text{2}}}{\text{ = 173pm}} \\
{\text{a = }}\dfrac{{{{2 \times 173}}}}{{\sqrt {\text{3}} }} \\
{\text{a = 200pm}} \\
\]
Note: The contribution of each corner atoms is \[{\dfrac{{\text{1}}}{{\text{8}}}^{{\text{th}}}}\] of a total atom. So, the contribution of all eight atoms is, \[\dfrac{{\text{1}}}{{\text{8}}} \times 8 = 1\].
For the body center atoms, the contribution of that atom is a total of a total atom.
Total number of atoms is 2 in a B.C.C lattice.
Formula used: Density = \[\dfrac{{(M \times z)}}{{(N \times {a^3})}}\]= \[\dfrac{{(molecular{\text{ }}weight){\text{ (}}number{\text{ }}of{\text{ }}atoms{\text{ }}per{\text{ }}cell){\text{ }}}}{{{{(edge{\text{ }}length)}^3}{\text{ (}}Avogadro{\text{ }}number)}}\]
Complete step by step answer:
The structure is shown below,

We know that,
Density = (mass)/(volume)
Applying this concept for calculating the density of the unite cell, we get,
Density = \[\dfrac{{(M \times z)}}{{(N \times {a^3})}}\]
= \[\dfrac{{(molecular{\text{ }}weight){\text{ (}}number{\text{ }}of{\text{ }}atoms{\text{ }}per{\text{ }}cell){\text{ }}}}{{{{(edge{\text{ }}length)}^3}{\text{ (}}Avogadro{\text{ }}number)}}\]
= \[7.2 = \dfrac{{M \times 2}}{{{{(288 \times {{10}^{10}})}^3} \times 6.022 \times {{10}^{23}}}}\]
Hence,
\[
7.2 = \dfrac{{M \times 2}}{{{{(288 \times {{10}^{10}})}^3} \times 6.022 \times {{10}^{23}}}} \\
M = \dfrac{{7.2 \times {{(288 \times {{10}^{10}})}^3} \times 6.022 \times {{10}^{23}}}}{2} \\
M = 51.77 \\
\]
Therefore, the molecular weight of the element is \[51.77gm/mol\].
Additional information:
In the B.C.C the closest distance between two atoms in the unit lattice is\[\dfrac{{\sqrt {\text{3}} a}}{2}\]. Where a is the edge length of the unit lattice.
Now according to this formula \[\dfrac{{\sqrt {\text{3}} a}}{{\text{2}}}{\text{ = 173pm}}\]
Therefore, the edge length is,
\[
\dfrac{{\sqrt {{\text{3a}}} }}{{\text{2}}}{\text{ = 173pm}} \\
{\text{a = }}\dfrac{{{{2 \times 173}}}}{{\sqrt {\text{3}} }} \\
{\text{a = 200pm}} \\
\]
Note: The contribution of each corner atoms is \[{\dfrac{{\text{1}}}{{\text{8}}}^{{\text{th}}}}\] of a total atom. So, the contribution of all eight atoms is, \[\dfrac{{\text{1}}}{{\text{8}}} \times 8 = 1\].
For the body center atoms, the contribution of that atom is a total of a total atom.
Total number of atoms is 2 in a B.C.C lattice.
Recently Updated Pages
Types of Solutions in Chemistry: Explained Simply

JEE Main Mock Test 2025-26: Principles Related To Practical

JEE Main 2025-26 Organic Compounds Containing Nitrogen Mock Test

JEE Main 2025-26 Mock Test: Organic Compounds Containing Oxygen

JEE Main 2025-26 Redox Reactions & Electro Mock Test

JEE Main Solutions Mock Test 1-2 (2025-26): Free Practice & Answers

Trending doubts
JEE Main 2026: Session 1 Results Out and Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

Ideal and Non-Ideal Solutions Explained for Class 12 Chemistry

JEE Main Participating Colleges 2026 - A Complete List of Top Colleges

Degree of Dissociation: Meaning, Formula, Calculation & Uses

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

Other Pages
CBSE Class 12 Chemistry Question Paper 2026 PDF Download (All Sets) with Answer Key

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The D And F Block Elements - 2025-26

JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 2 Electrochemistry - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions - 2025-26




