
An element crystallizes in bcc structure. The edge length of its unit cell is 288 pm. If the density of the crystal is 7.2 g \[cm ^{- 3}\]. what is the atomic mass \[\;\left( {in{\text{ }}g/mol} \right)\] of the element?
A. 51.8
B. 103.6
C. 25.9
D. 207.2
Answer
232.8k+ views
Hint: Body-centered cubic unit cell is one kind of cubic lattice. In this cubic lattice each unit cell contains one atom at its body center. And contains one atom at the eight corners of the unit cell.
Formula used: Density = \[\dfrac{{(M \times z)}}{{(N \times {a^3})}}\]= \[\dfrac{{(molecular{\text{ }}weight){\text{ (}}number{\text{ }}of{\text{ }}atoms{\text{ }}per{\text{ }}cell){\text{ }}}}{{{{(edge{\text{ }}length)}^3}{\text{ (}}Avogadro{\text{ }}number)}}\]
Complete step by step answer:
The structure is shown below,
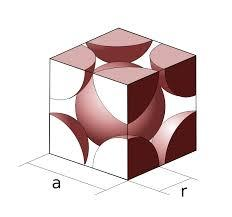
We know that,
Density = (mass)/(volume)
Applying this concept for calculating the density of the unite cell, we get,
Density = \[\dfrac{{(M \times z)}}{{(N \times {a^3})}}\]
= \[\dfrac{{(molecular{\text{ }}weight){\text{ (}}number{\text{ }}of{\text{ }}atoms{\text{ }}per{\text{ }}cell){\text{ }}}}{{{{(edge{\text{ }}length)}^3}{\text{ (}}Avogadro{\text{ }}number)}}\]
= \[7.2 = \dfrac{{M \times 2}}{{{{(288 \times {{10}^{10}})}^3} \times 6.022 \times {{10}^{23}}}}\]
Hence,
\[
7.2 = \dfrac{{M \times 2}}{{{{(288 \times {{10}^{10}})}^3} \times 6.022 \times {{10}^{23}}}} \\
M = \dfrac{{7.2 \times {{(288 \times {{10}^{10}})}^3} \times 6.022 \times {{10}^{23}}}}{2} \\
M = 51.77 \\
\]
Therefore, the molecular weight of the element is \[51.77gm/mol\].
Additional information:
In the B.C.C the closest distance between two atoms in the unit lattice is\[\dfrac{{\sqrt {\text{3}} a}}{2}\]. Where a is the edge length of the unit lattice.
Now according to this formula \[\dfrac{{\sqrt {\text{3}} a}}{{\text{2}}}{\text{ = 173pm}}\]
Therefore, the edge length is,
\[
\dfrac{{\sqrt {{\text{3a}}} }}{{\text{2}}}{\text{ = 173pm}} \\
{\text{a = }}\dfrac{{{{2 \times 173}}}}{{\sqrt {\text{3}} }} \\
{\text{a = 200pm}} \\
\]
Note: The contribution of each corner atoms is \[{\dfrac{{\text{1}}}{{\text{8}}}^{{\text{th}}}}\] of a total atom. So, the contribution of all eight atoms is, \[\dfrac{{\text{1}}}{{\text{8}}} \times 8 = 1\].
For the body center atoms, the contribution of that atom is a total of a total atom.
Total number of atoms is 2 in a B.C.C lattice.
Formula used: Density = \[\dfrac{{(M \times z)}}{{(N \times {a^3})}}\]= \[\dfrac{{(molecular{\text{ }}weight){\text{ (}}number{\text{ }}of{\text{ }}atoms{\text{ }}per{\text{ }}cell){\text{ }}}}{{{{(edge{\text{ }}length)}^3}{\text{ (}}Avogadro{\text{ }}number)}}\]
Complete step by step answer:
The structure is shown below,

We know that,
Density = (mass)/(volume)
Applying this concept for calculating the density of the unite cell, we get,
Density = \[\dfrac{{(M \times z)}}{{(N \times {a^3})}}\]
= \[\dfrac{{(molecular{\text{ }}weight){\text{ (}}number{\text{ }}of{\text{ }}atoms{\text{ }}per{\text{ }}cell){\text{ }}}}{{{{(edge{\text{ }}length)}^3}{\text{ (}}Avogadro{\text{ }}number)}}\]
= \[7.2 = \dfrac{{M \times 2}}{{{{(288 \times {{10}^{10}})}^3} \times 6.022 \times {{10}^{23}}}}\]
Hence,
\[
7.2 = \dfrac{{M \times 2}}{{{{(288 \times {{10}^{10}})}^3} \times 6.022 \times {{10}^{23}}}} \\
M = \dfrac{{7.2 \times {{(288 \times {{10}^{10}})}^3} \times 6.022 \times {{10}^{23}}}}{2} \\
M = 51.77 \\
\]
Therefore, the molecular weight of the element is \[51.77gm/mol\].
Additional information:
In the B.C.C the closest distance between two atoms in the unit lattice is\[\dfrac{{\sqrt {\text{3}} a}}{2}\]. Where a is the edge length of the unit lattice.
Now according to this formula \[\dfrac{{\sqrt {\text{3}} a}}{{\text{2}}}{\text{ = 173pm}}\]
Therefore, the edge length is,
\[
\dfrac{{\sqrt {{\text{3a}}} }}{{\text{2}}}{\text{ = 173pm}} \\
{\text{a = }}\dfrac{{{{2 \times 173}}}}{{\sqrt {\text{3}} }} \\
{\text{a = 200pm}} \\
\]
Note: The contribution of each corner atoms is \[{\dfrac{{\text{1}}}{{\text{8}}}^{{\text{th}}}}\] of a total atom. So, the contribution of all eight atoms is, \[\dfrac{{\text{1}}}{{\text{8}}} \times 8 = 1\].
For the body center atoms, the contribution of that atom is a total of a total atom.
Total number of atoms is 2 in a B.C.C lattice.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




