
Alkyl cyanides when react with Grignard reagent, the product on hydrolysis found is
A. Aldehyde
B. Ketone
C. Alcohol
D. Acid
Answer
240.3k+ views
Hint: Toluene is a transparent, colourless liquid that, when heated to room temperature, turns into a vapour. Toluene is a naturally occurring component of crude oil and is used in the production of paints, lacquers, explosives (TNT), and glues, in addition to oil refining. Toluene exposure can lead to a variety of symptoms, including irritation of the eyes and nose, exhaustion, headache, dilated pupils, tears, anxiety, muscle fatigue, sleeplessness, and nerve damage.
Complete Step by Step Answer:
Grignard reagent has two parts. In the Grignard reagent the alkyl part acts as a nucleophile that is electron efficient species and wants to donate its electrons. In this reaction in the first step the alkyl part of the Grignard reagent attacks the carbon atom of the alkyl cyanide and the metal part of the Grignard reagent that is magnesium part connects with the nitrogen atom of cyanide.
In the next step water attacks the carbon atom and makes ketone along with ammonia as a side product. The reaction can be given as follows:
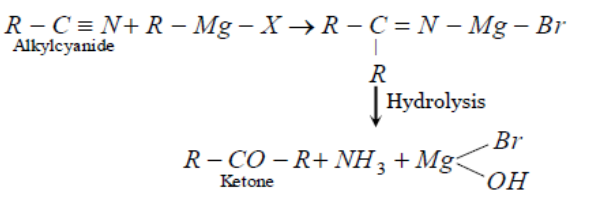
Thus we can write that Alkyl cyanides when react with Grignard reagent, the product on hydrolysis found is ketone.
Thus the correct option is B.
Note: Grignard reagent is alkyl or aryl magnesium halide type of compound. In Grignard reagent the alkyl or aryl part acts as a nucleophile and the metal part gets positive charge. Grignard reagents can convert aldehyde and ketone to alcohol. It can also convert acid to alcohol. In these Grignard reagents the metal that is magnesium acts as a hard species.
Complete Step by Step Answer:
Grignard reagent has two parts. In the Grignard reagent the alkyl part acts as a nucleophile that is electron efficient species and wants to donate its electrons. In this reaction in the first step the alkyl part of the Grignard reagent attacks the carbon atom of the alkyl cyanide and the metal part of the Grignard reagent that is magnesium part connects with the nitrogen atom of cyanide.
In the next step water attacks the carbon atom and makes ketone along with ammonia as a side product. The reaction can be given as follows:
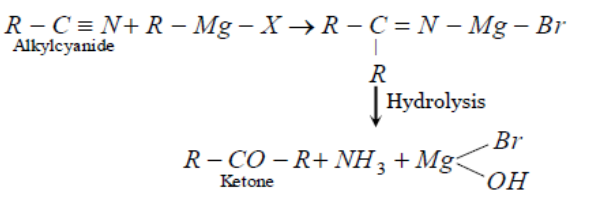
Thus we can write that Alkyl cyanides when react with Grignard reagent, the product on hydrolysis found is ketone.
Thus the correct option is B.
Note: Grignard reagent is alkyl or aryl magnesium halide type of compound. In Grignard reagent the alkyl or aryl part acts as a nucleophile and the metal part gets positive charge. Grignard reagents can convert aldehyde and ketone to alcohol. It can also convert acid to alcohol. In these Grignard reagents the metal that is magnesium acts as a hard species.
Recently Updated Pages
Types of Solutions in Chemistry: Explained Simply

JEE Main Mock Test 2025-26: Principles Related To Practical

JEE Main 2025-26 Organic Compounds Containing Nitrogen Mock Test

JEE Main 2025-26 Mock Test: Organic Compounds Containing Oxygen

JEE Main 2025-26 Redox Reactions & Electro Mock Test

JEE Main Solutions Mock Test 1-2 (2025-26): Free Practice & Answers

Trending doubts
JEE Main 2026: Session 1 Results Out and Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

Ideal and Non-Ideal Solutions Explained for Class 12 Chemistry

JEE Main Participating Colleges 2026 - A Complete List of Top Colleges

Clemmensen and Wolff Kishner Reductions Explained for JEE & NEET

Degree of Dissociation: Meaning, Formula, Calculation & Uses

Understanding the Angle of Deviation in a Prism

Other Pages
NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The D And F Block Elements - 2025-26

JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 2 Electrochemistry - 2025-26

JEE Advanced 2026 - Exam Date (Released), Syllabus, Registration, Eligibility, Preparation, and More

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions - 2025-26




