
A gas expands under constant pressure $P$ from volume ${V_1}$ to ${V_2}$ , the work done by the gas is:
A. $P({V_2} - {V_1})$
B. Zero
C. $P({V_1} - {V_2})$
D. $P\left( {\dfrac{{{V_1}{V_2}}}{{{V_2} - {V_1}}}} \right)$
Answer
232.8k+ views
Hint:In the case, when a problem is based on thermodynamics, we know that all the parameters such as pressure, temperature, volume, work done, etc., vary with the given conditions of the system and surroundings hence, use different processes given in thermodynamics to state the answer for the given situation.
Complete step by step solution:
Isobaric process in thermodynamics is a process during which the pressure of a system remains constant that’s why it is also referred to as a constant-pressure process.
$\Delta P = 0$
Since a gas expands under constant pressure $P$ from volume ${V_1}$ to ${V_2}$ , therefore the process is Isobaric.
The Above given can be represented graphically as: -
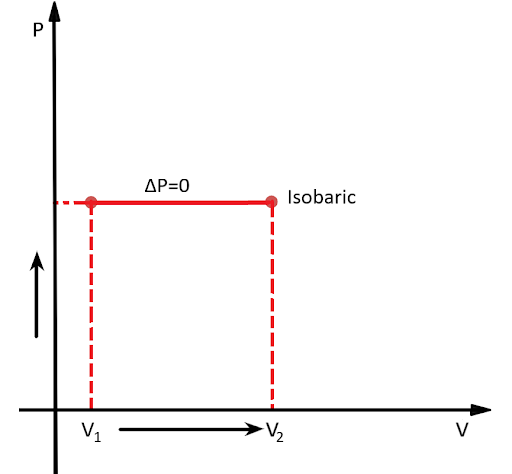
We know that in any thermodynamic process work done is always equal to the area covered by the $P - V$ curve with the volume axis. Therefore, from the above-plotted graph, Pressure-Volume work in thermodynamics is defined as: -
At constant pressure,
$\text{Work done} = W = P\Delta V$
It can also be written as: -
$\text{Work done} = W = P({V_2} - {V_1})$
Thus, when a gas expands under constant pressure, the work done by the gas is $P({V_2} - {V_1})$.
Hence, the correct option is A.
Note: Since this is a problem of multiple-choice question (theory-based) hence, it is essential that given options are analyzed very carefully to give a precise explanation. While writing an explanation of this kind of conceptual problem, always keep in mind to provide the exact reasons in support of your explanation.
Complete step by step solution:
Isobaric process in thermodynamics is a process during which the pressure of a system remains constant that’s why it is also referred to as a constant-pressure process.
$\Delta P = 0$
Since a gas expands under constant pressure $P$ from volume ${V_1}$ to ${V_2}$ , therefore the process is Isobaric.
The Above given can be represented graphically as: -

We know that in any thermodynamic process work done is always equal to the area covered by the $P - V$ curve with the volume axis. Therefore, from the above-plotted graph, Pressure-Volume work in thermodynamics is defined as: -
At constant pressure,
$\text{Work done} = W = P\Delta V$
It can also be written as: -
$\text{Work done} = W = P({V_2} - {V_1})$
Thus, when a gas expands under constant pressure, the work done by the gas is $P({V_2} - {V_1})$.
Hence, the correct option is A.
Note: Since this is a problem of multiple-choice question (theory-based) hence, it is essential that given options are analyzed very carefully to give a precise explanation. While writing an explanation of this kind of conceptual problem, always keep in mind to provide the exact reasons in support of your explanation.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding Uniform Acceleration in Physics

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Laws of Motion Class 11 Physics Chapter 4 CBSE Notes - 2025-26

Waves Class 11 Physics Chapter 14 CBSE Notes - 2025-26

Mechanical Properties of Fluids Class 11 Physics Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Physics Chapter 11 CBSE Notes - 2025-26

Units And Measurements Class 11 Physics Chapter 1 CBSE Notes - 2025-26




