
1, 3-butadiene reacts with ethylene to form
A. Benzene
B. Cyclohexane
C. Cyclohexene
D. 2, 3 dimethyl butane
Answer
233.1k+ views
Hint: Hydrocarbons containing two double bonds are called dienes. These are also called alkadienes. The reaction of 1,3-butadiene with ethylene involves the formation of a six-membered ring by the 1,4-addition of an alkene to a conjugated diene.
Complete Step by Step Answer:
Hydrocarbons containing two double bonds are called dienes. These are also called alkadienes.
These compounds are isomeric with alkynes.
1,3-butadiene is a conjugated diene or a single bond that intervenes with two double bonds. They have an arrangement of alternate single and double bonds.
The reaction of 1,3-butadiene with ethylene involves the formation of a six-membered ring by the 1,4-addition of an alkene to a conjugated diene. This reaction is known as the Diels-Alder reaction.
Alkene used is known as a dienophile and the product is known as Diels-Alder adduct. It is a cycloaddition reaction. Diels-Alder reaction is a 1,4 addition of ethene to 1,3-butadiene and also a 1,2 addition of butadiene to ethene. It is known as [4 + 2] cycloaddition leading to the formation of a six-membered ring. This six-membered ring is cyclohexene.
The reaction happens as follows:
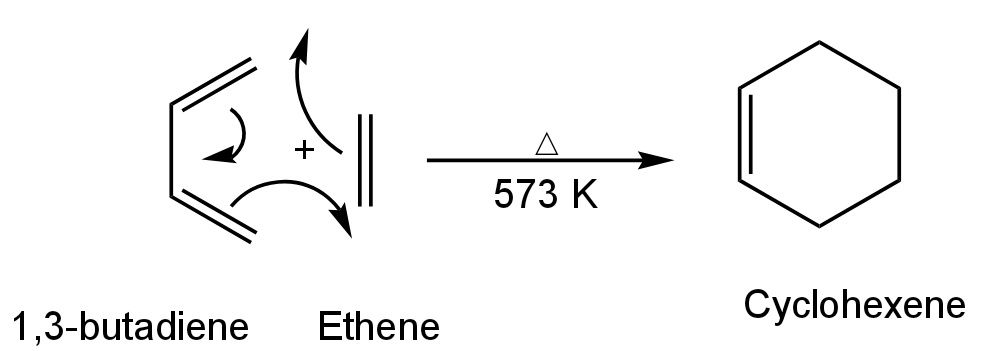
Image: Diels-Alder reaction
So, 1, 3-butadiene reacts with ethylene to form cyclohexane.
So, option C is correct.
Note: Ethene and other simple alkenes normally are weak dienophiles and react with 1,3-butadiene only under drastic conditions and in low yield. But, when the hydrogen atoms in the diene are replaced with various electron-attracting groups such as chlorine or bromine, electron-donating groups the reaction happens on its own.
Complete Step by Step Answer:
Hydrocarbons containing two double bonds are called dienes. These are also called alkadienes.
These compounds are isomeric with alkynes.
1,3-butadiene is a conjugated diene or a single bond that intervenes with two double bonds. They have an arrangement of alternate single and double bonds.
The reaction of 1,3-butadiene with ethylene involves the formation of a six-membered ring by the 1,4-addition of an alkene to a conjugated diene. This reaction is known as the Diels-Alder reaction.
Alkene used is known as a dienophile and the product is known as Diels-Alder adduct. It is a cycloaddition reaction. Diels-Alder reaction is a 1,4 addition of ethene to 1,3-butadiene and also a 1,2 addition of butadiene to ethene. It is known as [4 + 2] cycloaddition leading to the formation of a six-membered ring. This six-membered ring is cyclohexene.
The reaction happens as follows:

Image: Diels-Alder reaction
So, 1, 3-butadiene reacts with ethylene to form cyclohexane.
So, option C is correct.
Note: Ethene and other simple alkenes normally are weak dienophiles and react with 1,3-butadiene only under drastic conditions and in low yield. But, when the hydrogen atoms in the diene are replaced with various electron-attracting groups such as chlorine or bromine, electron-donating groups the reaction happens on its own.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




