




What Are Substrates? Meaning, Types, and Their Role in Reactions
The substrate is an umbrella term, which has a variable significance in different disciplines. From the chemical point of view, the core concept of substrates may be understood as the raw materials which, under an enzyme-catalysed reaction, yield a desirable product. The product thus produced can be prepared even without enzymes but the rate than would be slowed down by a million times.
With reference to ecology though, it may refer to the substratum on which an organism lives, but for microbiologists it is the chemical on which microbial organisms can feed and thrive. In the biochemical branch of science, the substrate is a substance on which an enzyme acts to produce the desired product by forming an intermediate.
Biochemical Substrate
The biochemical significance of a substrate is in enzyme catalysis. A biochemical substrate can be considered a raw material used to obtain a certain product. The chemical reaction, thus, occurring, is driven by an enzyme. An enzyme is a proteinaceous molecule of high molecular weight. It may have multiple sites, out of which one is for the attachment of substrate. The lock-key mechanism may explain the enzyme-substrate association, wherein the substrate is the key and the enzyme is the lock. Just like a key fits into a lock, the substrate fits into the active site of an enzyme to give a product.
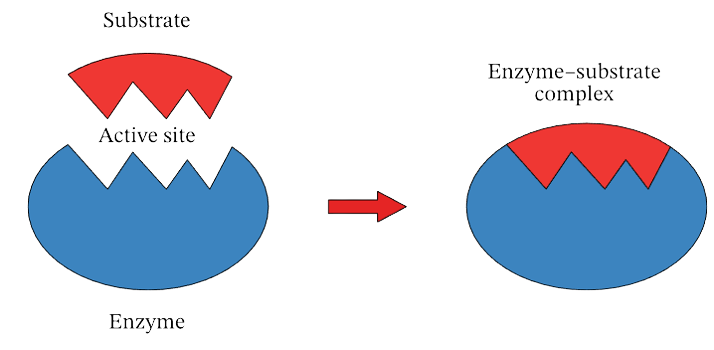
Enzyme catalysis depicting association of substrate with active site of enzyme
Enzyme Catalysis
Enzymes are usually proteinaceous molecules with the exception of ribozymes which are RNA molecules. They are biological catalysts of high molecular weight, ranging from $2000$ to more than $1$ million Dalton. These catalysts are sensitive to changes in temperature and pH. For most enzymes, the optimum temperature range lies between $298-310$ K and the optimum pH lies in the range of 5-7. Enzyme catalysis can be understood through adsorption and intermediate formation.
The substrate first diffuses and then adsorbs to bind to the enzyme's active site. It then reacts with the active groups, like $-NH_{2}$ , $-COOH$ , $-SH$ , $-OH$ , etc., present around the active site to form an activated complex. This complex finally dissociates to form a product. The product then desorbs and diffuses away from the enzyme. This reaction can be represented in the form of chemical equations as follows:
$E \ + \ S \ \rightarrow \ ES^{*}\\\\ES^{*} \rightarrow \ E \ + \ P$
Every enzyme has a specific condition under which it can work if that condition changes its working efficiency reduces even sometimes enzymes also get denatured as other proteins.
Substrate Specificity
Due to their chemical structure, enzymes are highly specific. One enzyme may react with only certain substrates to yield a specific product. The nature of an enzyme to react with only certain substrates is known as substrate specificity. The size, structure, charges, polarity, and hydrophobicity of the substrate as well as the active site determine the substrate specificity.
Substrate in Other Domains
Substrates have diversified applications that vary from one field to another.
In ecology, substrates describe the surfaces on which different microorganisms, including plants, fungi, and algae, can exist. As a result, the rock can be considered a substrate for the algae living on it, while the algae itself can be said to be a substrate for any organism living on it.
In microbiology, the importance of substrates can be expressed as the source of nutrition and energy for the microorganism. A few examples of unusual substrates bacteria use for growth include carbon monoxide and other toxic environmental pollutants, spent wash, molasses and sewage.
Iron may be referred to as a substrate on which zinc is coated during galvanisation.
Summary
The word substrate holds different meanings and significance in ecological, biological and biochemical fields. The chemical definition of the substrate is related to enzyme catalysis. Enzymes are catalysts which react with substrates to form products. These catalysts are mostly proteinaceous, specific and highly sensitive to temperature and pH changes. In other regards, substrates are also the basic material for our biological processes, these may also be sources of nutrients required for growing a population, or a substrate may also refer to a substrate used for attachment.
List of Related Articles
- Enzymes
- Application of Enzymes
FAQs on Substrate in Chemistry: Complete Guide with Examples
1. What is a substrate in chemistry?
In chemistry, a substrate is the specific reactant molecule that is acted upon by a catalyst, such as an enzyme, in a chemical reaction. It is the substance that binds to the catalyst's active site and is subsequently converted into a different molecule, known as the product. In a broader sense, it can also refer to the surface on which a reaction takes place.
2. What are some common examples of substrates in chemical reactions?
Substrates are fundamental to many biological and industrial chemical processes. Here are a few key examples:
- In Digestion: Proteins are substrates for the enzyme pepsin in the stomach, which breaks them down into smaller peptides.
- In Cellular Respiration: Glucose is the primary substrate that enters the glycolysis pathway to be converted into pyruvate, releasing energy.
- In Fermentation: Sugars like glucose and sucrose are substrates for yeast enzymes, which convert them into ethanol and carbon dioxide.
- In Organic Synthesis: In the formation of soap (saponification), the fat or oil (triglyceride) is the substrate that reacts with a strong base like NaOH.
3. How is a substrate different from a reagent?
While both are reactants in a chemical reaction, the terms have distinct connotations. A reagent is a general term for any substance added to cause a chemical reaction. A substrate is more specific; it typically refers to the main molecule of interest that is being transformed by other reagents or, in biochemistry, the molecule that an enzyme specifically targets. For example, in the hydrogenation of ethene to ethane, ethene is the substrate and hydrogen (with a catalyst) is the reagent.
4. What is the role of a substrate in an enzyme-catalysed reaction?
In an enzyme-catalysed reaction, the substrate's role is to be the specific raw material that the enzyme converts into a product. The process begins when the substrate binds to a unique location on the enzyme called the active site. This binding forms a temporary enzyme-substrate complex. Within this complex, the enzyme lowers the activation energy required for the reaction, facilitating a rapid conversion of the substrate into products, which are then released.
5. Why is the 'lock and key' model often used to explain enzyme-substrate interaction?
The 'lock and key' model is an analogy used to explain the high specificity of enzymes. In this model, the enzyme's active site is represented as a 'lock' with a unique, rigid shape. The substrate is the 'key' that has a complementary shape. This explains why a specific enzyme, like lactase, can only act on its specific substrate, lactose, and not on other sugars. It highlights that the physical fit between the enzyme and substrate is crucial for the reaction to occur.
6. How does substrate concentration affect the rate of an enzyme-catalysed reaction?
Substrate concentration directly influences the reaction rate, but only up to a certain point. Initially, increasing the substrate concentration leads to a faster reaction rate because more substrate molecules are available to collide with and bind to the enzyme's active sites. However, the rate eventually plateaus when the enzymes become saturated. At this saturation point (known as Vmax), all active sites are occupied, and the reaction rate is limited by how fast the enzyme can process the substrate, not by the availability of the substrate.
7. What is the difference between a substrate and a coenzyme?
The primary difference lies in their function and fate during a reaction. A substrate is the reactant molecule that is consumed and permanently converted into a product. In contrast, a coenzyme is a non-protein 'helper' molecule that binds to the enzyme and assists in the reaction, often by transferring electrons or functional groups. The coenzyme is not consumed; it is regenerated and can be used in multiple reaction cycles. Many vitamins are precursors to coenzymes.
8. What is a chromogenic substrate and what is its main application?
A chromogenic substrate is a compound that is initially colourless but produces a coloured product when an enzyme acts upon it. Its primary application is as a visual indicator in diagnostic tests and laboratory research. The appearance of colour confirms the presence and activity of a specific enzyme. A well-known example is X-gal, used in genetic engineering, which turns blue in the presence of the enzyme β-galactosidase, allowing for easy identification of successful gene cloning.























