




How to Identify Polar Compounds in Chemistry
Molecules or their chemical groups have an electric dipole moment with a negatively charged end and a positively charged end when they are polar, which in chemistry is the separation of electric charge. This separation of electric charge is known as polarity. In a bond, this polarity difference leads to unequal sharing of electrons between the atoms, as electrons will be pulled closer to the atom with the higher electronegativity. The difference in electronegativity determines the nature of chemical compounds. Compounds are classified as polar, non-polar, and ionic compounds based on electronegativities. Polar covalent compounds conduct electricity.
Knowing What Are Polar Compounds
Polar compounds are chemical substances that are bound together by polar covalent bonds. A chemical species known as a 'polar compound' is one that has two or more atoms that are held together by covalent bonds that are polar in character and that share electrons unevenly. When two atoms are joined together by covalent bonds then shared pairs of electrons are shifted toward atoms that are more electronegative in nature. The electronegativity of an atom is defined as the tendency to attract electrons. Hence, more electronegative atoms attract electrons and increase the electron density towards itself.
Characteristics of Polar Compounds
Non-bonding of an electron pair in a molecular orbital also accounts for the polarity of a molecule.
Polar compounds always have some net value of dipole moment. The presence of opposing charges in a molecule results in a net dipole.
Polar substances like distilled water and ethanol cannot conduct because the dipoles are locked within the molecules by covalent bonds. Unless the polar bond is broken heterolytically to form two oppositely charged ions, a polar compound cannot conduct electricity.
Polar molecules having covalent bonds possess high boiling and melting points due to the presence of dipole-dipole attraction, as compared to molecules that are joined by different forces of attraction like van der Waal forces.
The forces of attraction are arranged as
Ionic bonds > hydrogen bonds > dipole-dipole > van der Waal forces.
Estimating Factors That Determine the Polarity of Compounds
Symmetry of a compound- Symmetry is inversely proportional to polarity. The more the symmetry of a molecule, the less polarity. The more symmetrical arrangements lead to the cancellation of the dipole moment. This reduces the polarity of the molecules.
Number of identical atoms- The presence of identical atoms nullifies the electronegativity difference and ultimately invalidates the polarity.
Number of lone pairs of electrons- Lone pairs are defined as electrons that are present in pairs around the atoms and do not participate in bonding with other atoms present for bonding. The presence of lone pairs around the central atoms enhances the chances of polarity by localisation of electrons around the atoms.
Shape of a molecule- The shape of a molecule determines the position of atoms and direction of the dipole moment because the dipole moment being vector quantity always moves from positively charged atoms to negatively charged atoms. Hence, it acts as a contributing factor toward polarity.
Some Examples of Polar Compounds
A molecule may be polar or nonpolar. A nonpolar molecule has a structure of its atoms lined up in a way that the orbital electrons in the outer region cancel out the electronegativity.
In general, pyramid-shaped and V-shaped molecules are said to be polar. Whereas the linear molecules are said to be non-polar in nature.
Water is said to be a polar molecule due to the difference in the electronegativities between the oxygen atom and the hydrogen. Oxygen is a highly electronegative atom when compared to hydrogen.
Fats, petrol, oil, and gasoline are said to be non-polar molecules as they do not dissolve in water and nonpolar is insoluble in water.
Glucose is one more example of a polar molecule based on the arrangement of the oxygen and hydrogen atoms in it.
More examples of Polar Compounds
Water - H2O
Ammonia - NH3
Sulphur dioxide - SO2
Hydrogen Sulphide - H2S
Ethanol -C2H6O
Non Polar Compounds
Any of the noble gases: He, Ne, Ar, Kr, Xe (These are atoms, not technically molecules.) Any of the homonuclear diatomic elements: H2, N2, O2, Cl2(These are truly nonpolar molecules.)
Carbon dioxide - CO2
Benzene -C6H6
Carbon tetrachloride - CCl4
Methane - CH4
Ethylene - C2H4
Hydrocarbon liquids, such as gasoline and toluene
Most organic molecules.
Do Polar Covalent Compounds Conduct Electricity?
Since polar covalent compounds' molecules have charge separation between them and behave as ions in aqueous state, they are excellent electrical conductors. These polar molecules conduct electricity and can move about freely in a solution.
Total Polar Compounds
During continuous frying, peroxides and hydroperoxides produce total polar compounds (TPC), comprising short chain fatty acids, aldehydes, ketones, alcohol, and nonvolatile products, whose polarities are greater than those of triglycerides.
These factors, which give end products or fried oil an unfavourable colour, odour, and excessive viscosity, are mostly to blame for the quality degradation of deep-fried foods. Thus, it is hypothesised that TPC produced during the frying of edible oil is responsible for the negative impacts on bodily health.
The detrimental effects of frying oil on health have only recently come to light. According to a report, oxidised fat and deep-fried oils may have a disproportionate role in the development of metabolic syndrome. Inhibiting development rate, encouraging liver size, and attenuating detoxifying enzymes connected to defence mechanisms against in vivo lipid peroxidation are a few ways that frying oils may impair liver function. Furthermore, frying oil and its polar components may disrupt the expression of the genes involved in vitamin A metabolism in the embryonic liver and result in malformations in pregnant mice. To our knowledge, however, the cellular impacts of TPC (the primary ingredients in frying oils) have been underappreciated.
Difference Between Polar and Nonpolar Compounds
Gaseous Polar Compounds Examples
H2O- H2O has a slightly bent structure in which an oxygen atom is present in the central position. Here, oxygen being more electronegative pulls electrons towards itself. This creates a slightly positive charge at hydrogen atoms and a slightly negative charge at oxygen atoms. Oxygen due to the presence of two lone pairs of electrons increases the lone pair-lone pair repulsion and results in the bent shape of the molecule. This molecule exists in all three forms i.e., solid, liquid and gas.
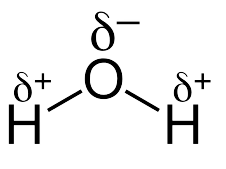
Structure of Water Molecule Showing Bent Shape or V-shape
CHCl3- This is known as Trichloromethane (chloroform). This comes under polar molecules due to the presence of net dipole because of electronegativity difference.

Structure of Trichloromethane Molecule
Key Features of Polar Compounds
Polar compounds are held together by covalent bonds.
There is unequal sharing of electrons between the atoms.
They should possess an electronegativity difference of more than 0.4.
Polarity of a molecule is affected by symmetry, lone pairs of electrons, and the shape of molecules.
FAQs on Polar Compounds: Definition, Properties & Examples
1. What is a polar compound according to the CBSE syllabus?
A polar compound is a molecule that has a net dipole moment due to the unequal sharing of electrons among its atoms. This occurs when atoms with different electronegativities form a covalent bond. The more electronegative atom attracts the shared electrons more strongly, creating a partial negative charge (δ-) on it and a partial positive charge (δ+) on the other atom. For a molecule to be polar, these individual bond dipoles must not cancel each other out due to an asymmetrical molecular shape.
2. How can you determine if a molecule is polar or non-polar?
To determine if a molecule is polar, you can follow these two key steps as per the 2025-26 NCERT curriculum:
- Check for Polar Bonds: First, see if the bonds within the molecule are polar. A bond is polar if the electronegativity difference between the two bonded atoms is significant (generally greater than 0.4).
- Analyse Molecular Geometry: Second, determine the molecule's three-dimensional shape using VSEPR theory. If the molecule contains polar bonds and has a symmetrical shape (like linear CO₂ or tetrahedral CCl₄), the individual bond dipoles cancel out, making the molecule non-polar. If the shape is asymmetrical (like the bent shape of H₂O or the trigonal pyramidal shape of NH₃), the dipoles do not cancel, and the molecule is polar.
3. What are the main differences between polar and non-polar compounds?
The main differences between polar and non-polar compounds stem from their electrical charge distribution:
- Dipole Moment: Polar compounds have a permanent, net dipole moment. Non-polar compounds have a zero net dipole moment.
- Solubility: Polar compounds typically dissolve in polar solvents like water ('like dissolves like'), whereas non-polar compounds dissolve in non-polar solvents like benzene or hexane.
- Intermolecular Forces: Polar molecules are held together by stronger dipole-dipole interactions, while non-polar molecules are held by weaker London dispersion forces. This generally gives polar compounds higher boiling points than non-polar compounds of similar mass.
- Molecular Shape: Polarity is often associated with asymmetrical shapes (e.g., bent, trigonal pyramidal), while non-polarity is associated with symmetrical shapes (e.g., linear, trigonal planar, tetrahedral with identical atoms).
4. Can you provide some common examples of polar and non-polar compounds?
Certainly. Here are some important examples you will find in your chemistry textbook:
- Examples of Polar Compounds: Water (H₂O), Ammonia (NH₃), Sulphur Dioxide (SO₂), Hydrogen Sulphide (H₂S), and Ethanol (C₂H₅OH). These molecules have asymmetrical shapes and a net dipole moment.
- Examples of Non-Polar Compounds: Carbon Dioxide (CO₂), Methane (CH₄), Carbon Tetrachloride (CCl₄), Benzene (C₆H₆), and all homonuclear diatomic molecules like O₂ and N₂. These molecules are symmetrical, causing their bond dipoles to cancel out.
5. Why is water (H₂O) a polar molecule, while carbon dioxide (CO₂) is non-polar, even though both contain polar bonds?
This is a classic example that highlights the importance of molecular geometry. While both H₂O and CO₂ have polar bonds (O-H and C=O respectively), their shapes lead to different overall polarities.
- Water (H₂O): The central oxygen atom has two lone pairs of electrons, which forces the molecule into a bent (V-shaped) geometry. This asymmetrical shape prevents the two O-H bond dipoles from cancelling each other out. Instead, they add up to create a strong net dipole moment, making water highly polar.
- Carbon Dioxide (CO₂): The central carbon atom has no lone pairs, resulting in a linear and symmetrical shape. The two C=O bond dipoles are equal in strength and point in opposite directions, causing them to completely cancel each other out. This results in a zero net dipole moment, making CO₂ a non-polar molecule.
6. How does the presence of lone pair electrons on the central atom affect a molecule's polarity?
The presence of lone pairs on a central atom is a crucial factor in determining polarity. According to VSEPR theory, lone pairs occupy more space than bonding pairs and exert greater repulsion. This often distorts the molecular geometry, making it asymmetrical. For example, in ammonia (NH₃), the lone pair on the nitrogen atom forces the molecule into a trigonal pyramidal shape. This asymmetry ensures that the individual N-H bond dipoles do not cancel, resulting in a net dipole moment and making NH₃ a polar molecule.
7. Why is carbon tetrachloride (CCl₄) non-polar, but chloroform (CHCl₃) is polar?
This comparison illustrates how even a small change in a molecule's composition can alter its polarity. Both molecules have a tetrahedral geometry, but their symmetry differs.
- Carbon Tetrachloride (CCl₄): The molecule is perfectly symmetrical. It has a central carbon atom bonded to four identical chlorine atoms. The four C-Cl bond dipoles are equal in magnitude and arranged symmetrically, so they cancel each other out, resulting in a zero net dipole moment.
- Chloroform (CHCl₃): Replacing one chlorine atom with a hydrogen atom breaks the symmetry. The C-H bond has a different polarity and bond length than the three C-Cl bonds. This imbalance prevents the bond dipoles from cancelling out, creating a net dipole moment and making the CHCl₃ molecule polar.
8. What is a dipole moment, and what is its role in determining the polarity of a compound?
A dipole moment (μ) is a quantitative measure of the polarity of a chemical bond or an entire molecule. It is a vector quantity, meaning it has both magnitude and direction. It arises from the separation of positive and negative charges.
Its role is fundamental: a molecule is classified as polar only if the vector sum of all its individual bond dipoles is non-zero. This resulting overall dipole is called the net dipole moment. If the bond dipoles are arranged symmetrically and cancel each other out, the net dipole moment is zero, and the molecule is non-polar.
























