
Class 12 Chemistry Amines Revision Notes Free PDF for NEET Preparation
Amines are a group of organic compounds that contain an amino group (-NH2) attached to the constituent carbon atoms. This classification of organic compounds comes with a specific set of physical and chemical properties. They are also formed when a hydrogen atom attached to the nitrogen atom of ammonia (NH3) is replaced by an alkyl group. To understand this classification and its fundamental concepts, refer to the Amines Class 12 notes prepared by the subject experts of Vedantu.
These notes cover all the important topics of this chapter so that NEET aspirants can complete revising this chapter faster. These notes will enable students to go through the fundamental theories related to amines faster so they can resolve doubts on their own.
Access NEET Revision Notes Chemistry Amines
Introduction
Amines are organic compounds made composed of amino group and one or more alkyl or aryl groups linked to the nitrogen atom. Amines have many functions in living things, including bioregulation, neurotransmission, and predator defence. Because of their high biological activity, several amines are used as medications and therapies.
Alkaloids are a type of physiologically active amine produced by plants to defend themselves against insects and other animals. While some alkaloids are used medicinally (as pain relievers), they are all dangerous and can cause death if ingested in large quantities.
Classification
One, two, or three alkyl or aryl groups are connected to nitrogen in primary, secondary, and tertiary amines, respectively.
Type | Examples |
Primary $({1^ \circ })$ $R - N{H_2}$ | 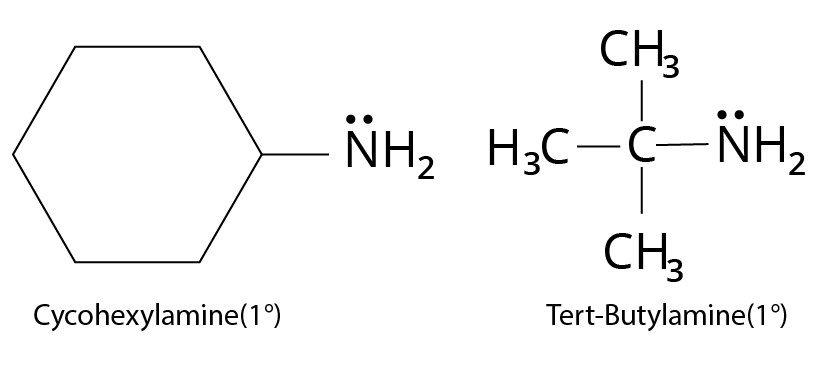
Structure of cyclohexylamine and tert-butylamine |
Secondary $({2^ \circ })$ ${R_2}NH$ | 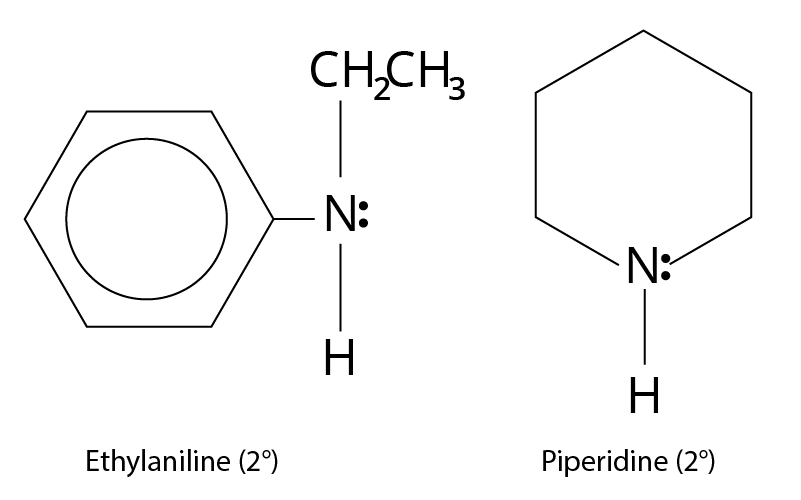
Structure of n-ethylamine and piperidine |
Tertiary $({3^ \circ })$ ${R_3}N$ | 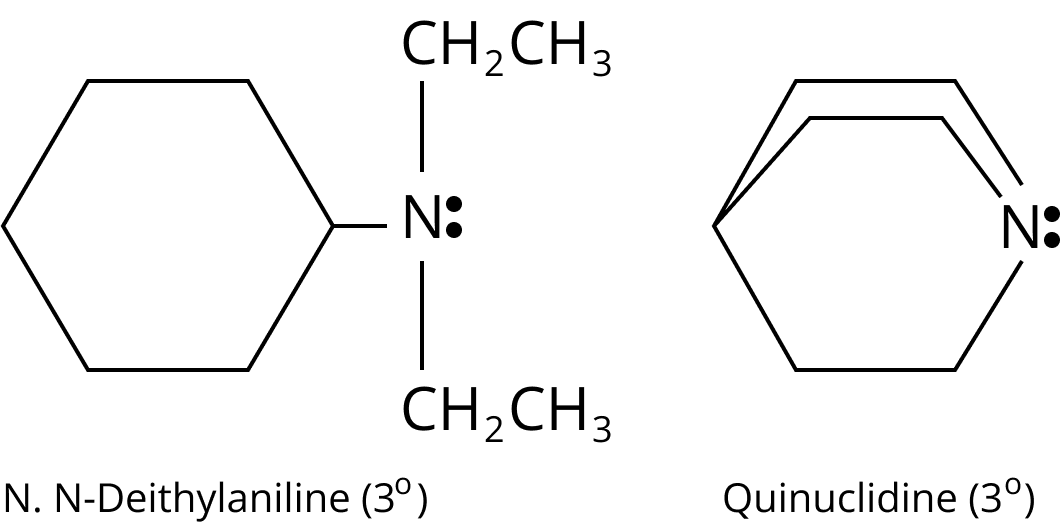
Structure of N,N-diethylaniline and Quinuclidine |
In quaternary ammonium ions, four alkyl or aryl bonds join a nitrogen atom. The nitrogen atom has a positive charge, much like in simple ammonium salts like ammonium chloride. The following is an example of quaternary ammonium salt:
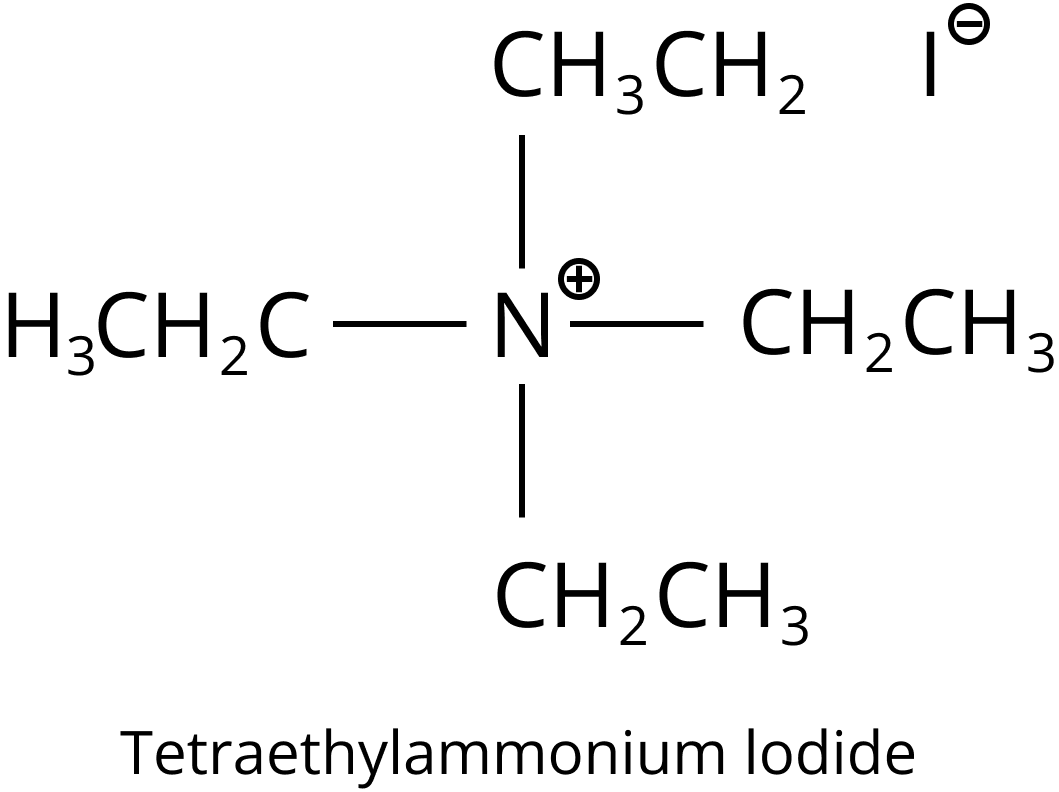
Structure of Tetraethylammonium Iodide
Structure of Amines
Ammonia's tetrahedral geometry is slightly distorted. A single pair of nonbonding electrons occupy one of the tetrahedral positions. In this geometry, the bulky lone pair compresses the $H - N - H$ bond angles to ${107^ \circ }$ from the "ideal" $s{p^3}$ bond angle of ${109.5^ \circ }$, which is represented by $s{p^3}$ nitrogen hybridization. Trimethylamine has less compression because the bulky methyl groups extend the angle slightly.
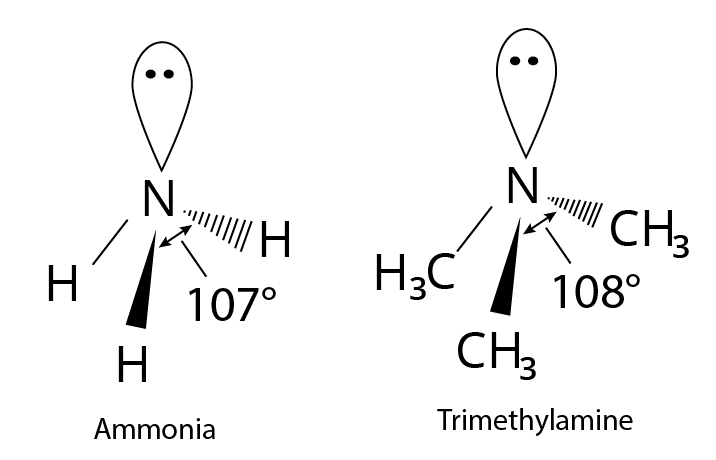
Structure of Ammonia and Trimethylamine
Non-superimposable is the mirror counterpart of a tetrahedral amine with three different substituents (including a lone pair). We can't normally resolve such an amine into two enantiomers since the enantiomers interconvert so quickly. During this process, known as nitrogen inversion, the lone pair moves from one side of the molecule to the other.
Since there is no lone pair to undergo nitrogen inversion in quarterary ammonium salts with asymmetric nitrogen atoms, inversion of configuration is not possible.
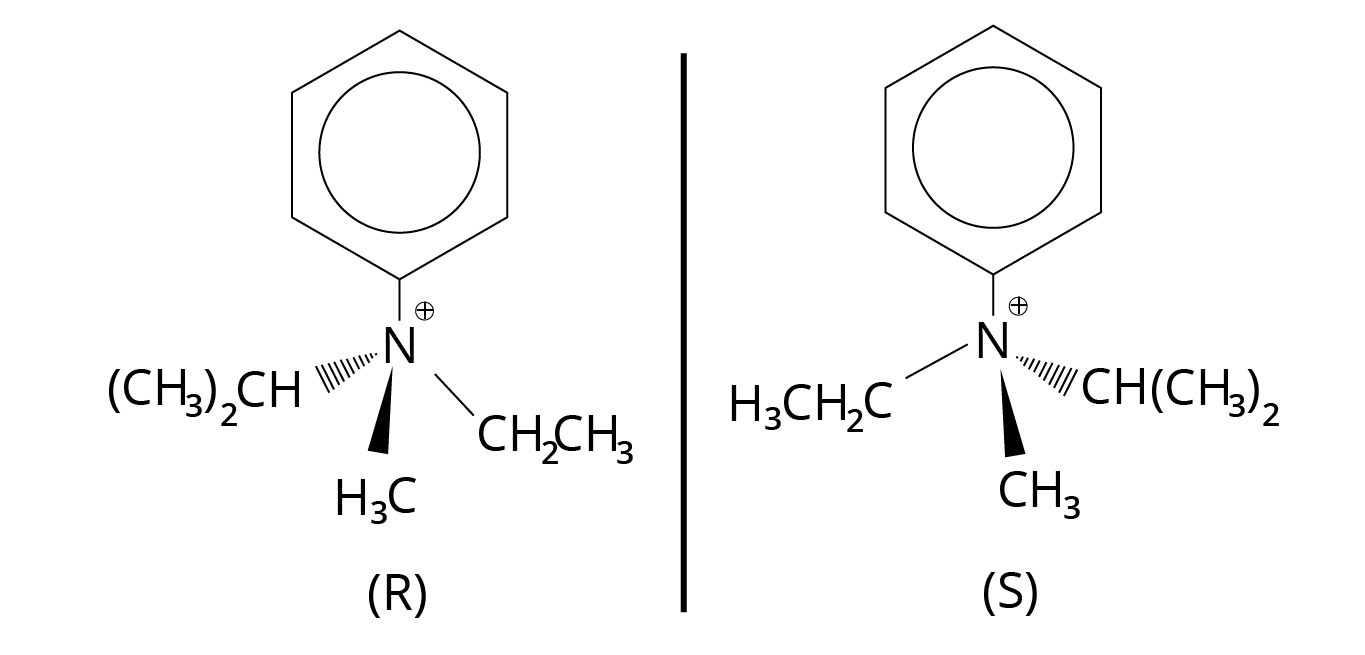
Structure of Quaternary Ammonium Salts
Amines that can't establish the $s{p^2}$ -hybrid transition state for nitrogen inversion also have chirality. If the nitrogen atom is confined in a small ring, it will be unable to attain the ${120^\circ} $ bond angles required for inversion.
Physical Properties
State
Lower aliphatic amines are odorless gaseous molecules that smell like fish. Liquid primary amines have three or more carbon atoms, while solid primary amines have more than three carbon atoms.
Aniline and other aryl amines are normally colourless, but they become coloured over time owing to air oxidation.
Dipole Moment
Since the significant dipole moment of the lone pair of electrons contributes to the dipole moments of the $C - H$ and $H - N$ bonds, amines are highly polar.
Solubility
Hydrogen bonds can be formed by $N - H$ bonds in primary and secondary amines. Because they lack $N - H$ bonds, pure tertiary amines cannot generate hydrogen bonds. However, hydrogen bonding from molecules with $O - H$ or $N - H$ bonds can be accepted.
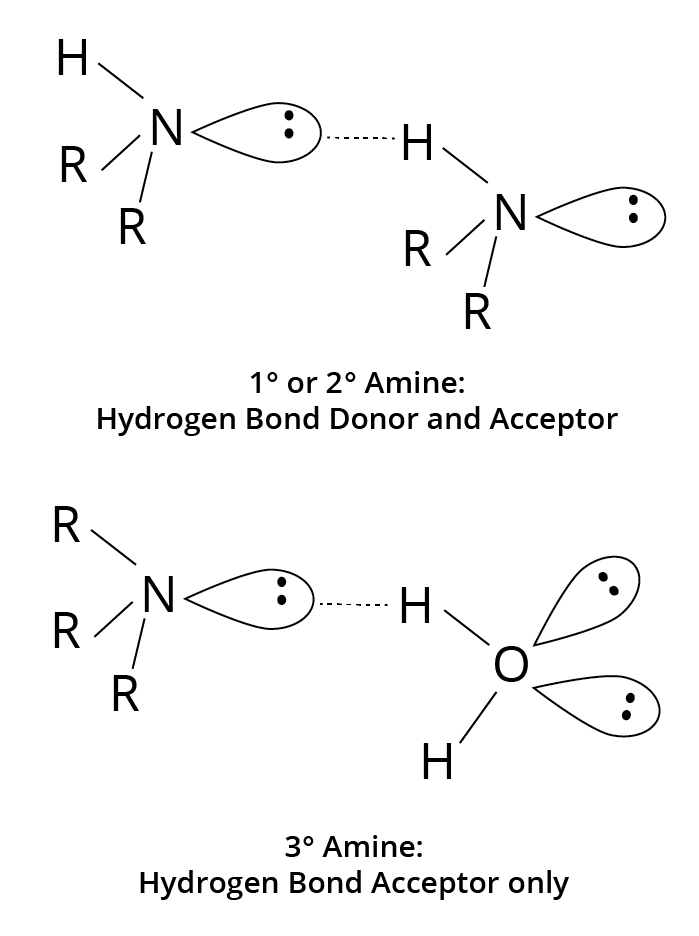
Hydrogen Bonding in Primary, Secondary and Tertiary Amines
Lower aliphatic amines are soluble in water due to their propensity to form hydrogen bonds with water. However, as the molar mass of amines rises, the size of the hydrophobic alkyl component grows larger, reducing solubility. Higher amines are largely insoluble in water.
Boiling Point
Since nitrogen is less electronegative than oxygen, the $N - H$ bond is less polar than the $O - H$ bond. As a result, hydrogen bonding between amines and alcohols of identical molecular weights are weaker. Primary and secondary amines have lower boiling temperatures than alcohols, but are higher than ethers with similar molecular weights. Because they lack hydrogen bonds, tertiary amines have lower boiling temperatures than primary and secondary amines with comparable molecular weights.
Compound | BP ${(^ \circ }C)$ | Type | Molecular weight |
${(C{H_3})_3}N$ | 3 | Tertiary Amine | 59 |
$C{H_3} - O - C{H_2} - C{H_3}$ | 8 | Ether | 60 |
$C{H_3} - NH - C{H_2} - C{H_3}$ | 37 | Secondary Amine | 59 |
$C{H_3}C{H_2}C{H_2} - N{H_2}$ | 48 | Primary Amine | 59 |
$C{H_3}C{H_2}C{H_2} - OH$ | 97 | Alcohol | 60 |
Preparation of Amines
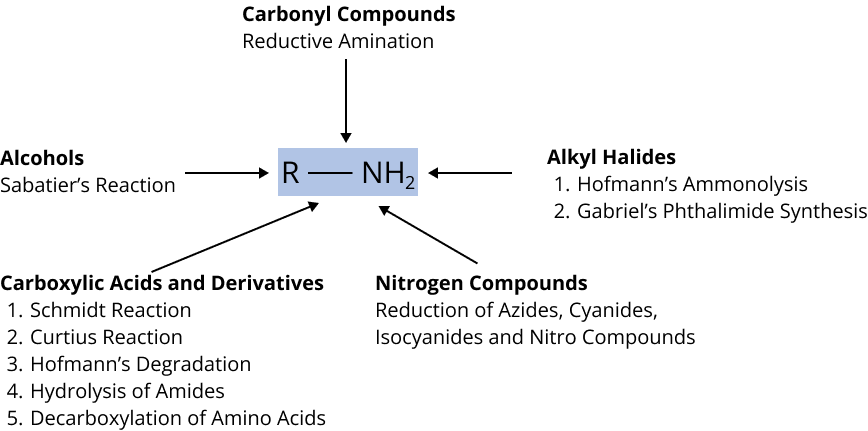
Preparation of Amines
Alkyl Halides
Hoffmann’s Ammonolysis Method
It's an ammonolysis case. An alkyl halide and an ethanolic ammonia solution are heated to ${100^ \circ }C$ in a sealed tube.
The process creates a combination of items.
Because it forms an alkene, tertiary alkyl halide is not recommended.
A bimolecular substitution $({S_{{N}}2})$ sets the stage for the reaction.
Gabriel’s Phthalimide Synthesis
Alkyl phthalimide is made by heating potassium phthalimide with alkyl halide after it has been treated with $KOH$. Only primary amine is produced by the subsequent hydrolysis. Alkyl phthalimide is also treated with hydrazine to enhance the yield of primary amine.
Example:

Reaction of Gabriel’s Phthalimide Synthesis
Grignard Reagent
After chloramine treatment, the primary amine is produced using the Grignard reagent or a trialkyl borane.
$RMgX + ClN{H_2} \to R - N{H_2} + MgXCl$
Alcohols
Sabatier Reaction
Alcohols and ammonia are heated under pressure in the presence of a catalyst like copper chromite or alumina. A mixture of goods is possible to gain.
Example:
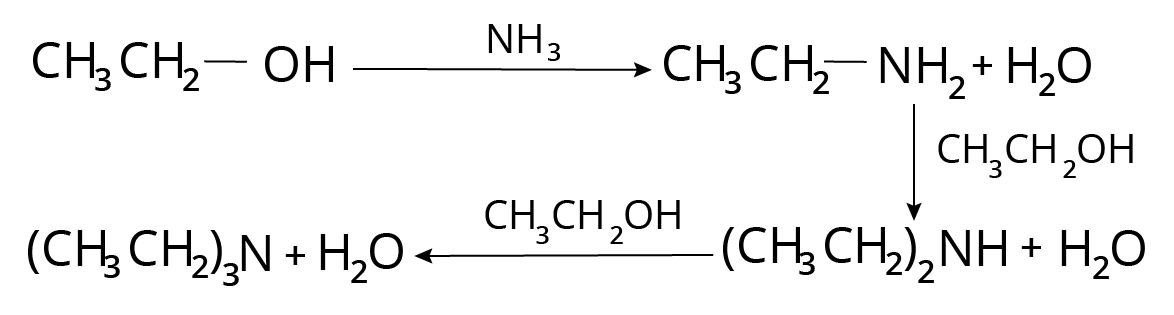
Example of Sabatier Reaction
Carbonyl Compounds
Reductive Amination
A number of aldehydes and ketones are converted to amines in the presence of ammonia.
Hydrogen $20 - 150\;atm$ over Raney nickel $40 - {150^\circ} C$ or sodium cyano hydrido borate, $NaB{H_3}CN$, can catalyse the reduction process.
Carboxylic Acids and Derivatives
Schmidt Reaction
A mixture of carboxylic acid and hydrazoic acid is treated with cold concentrated sulphuric acid to form primary amine.
$RCOOH + {N_3}H\xrightarrow[{{H_2}S{O_4}}]{{Cold\;conc.}}RN{H_2} + C{O_2} + {N_2}$
Example:
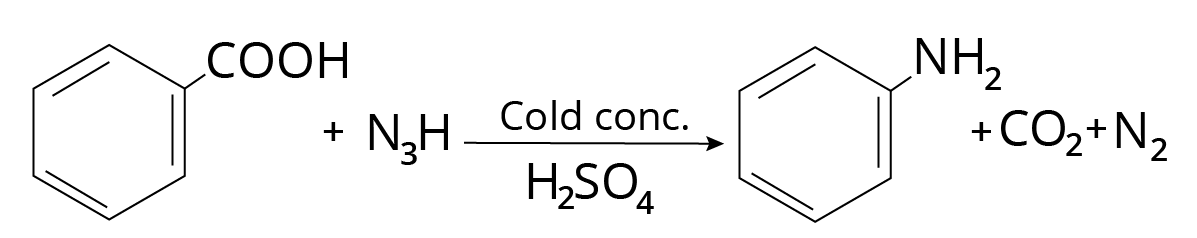
Schmidt Reaction
Curtius Reaction
In this procedure, the acyl azide is pyrolyzed to produce isocyanates. Amine is formed as a result of the following hydrolysis.
Example:
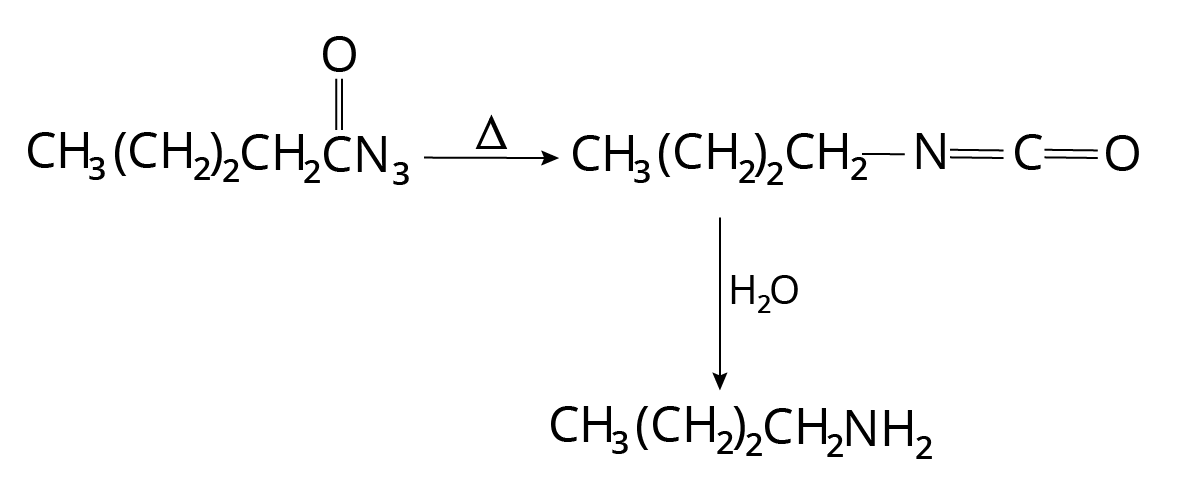
Curtius Reaction
In the first step nitrogen gas evolves and carbon dioxide is produced along with the amine in the second step.
Hofmann’s Degradation of Amides
The amide is warmed with bromine and a strong aqueous KOH or NaOH solution.
The final product is a primary amine with one fewer carbon atoms than amide.
Alkyl isocyanate is intermediate, and the rearrangement is intramolecular.
Example:
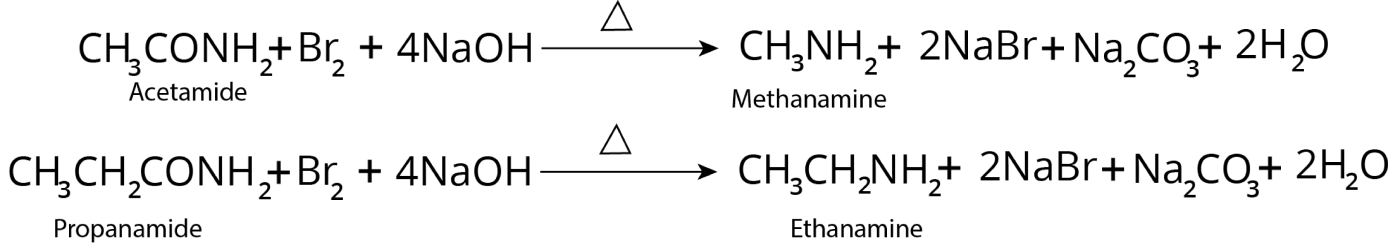
Reaction of Hofmann Degradation
Hydrolysis of Amides and Isocyanides
Primary amine is given on the hydrolysis of N-substituted amide and isocyanide.
Example:

Hydrolysis of Amides
Example:
$R - NC\xrightarrow[\Delta ]{{KOH,\;{H_2}O}}R - N{H_2} + HCOOK$
Hydrolysis of Isocyantes also yields amines.
$R - N = C = O\xrightarrow[\Delta ]{{{H_3}{O^ + }}}R - N{H_2}$
Decarboxylation of Amino Acids
Primary amine is formed when amino acids are heated with barium hydroxide. On heating, quaternary ammonium hydroxide decomposes into alcohol if there is no $\beta $ -hydrogen in the alkyl group, but if there is, Hofmann elimination occurs.
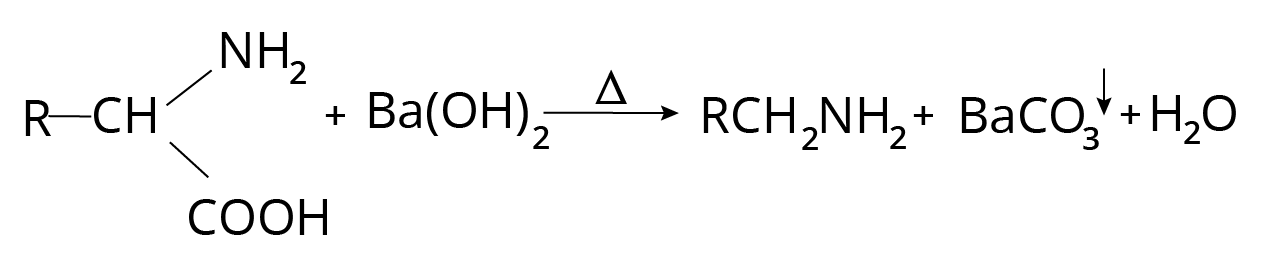
Degradation Reaction of Amino Acids
Nitrogen Compounds
Reduction of Cyanides, Isocyanides, Oximes, Imines
Nitrile $( - CN)$ , oxime $( = NOH)$ , imine $( = NH)$ , enamine etc, on catalytic reduction with ${H_2}$ , nickel gives corresponding amine.
Example:
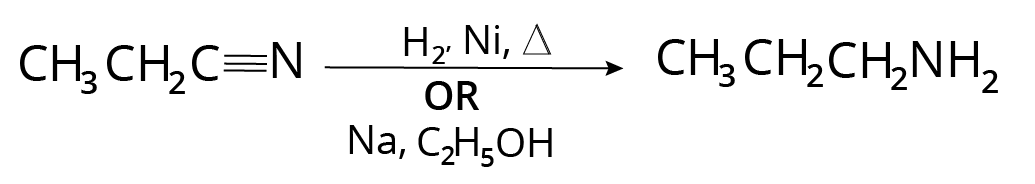
Reaction of Cyanide
Example:
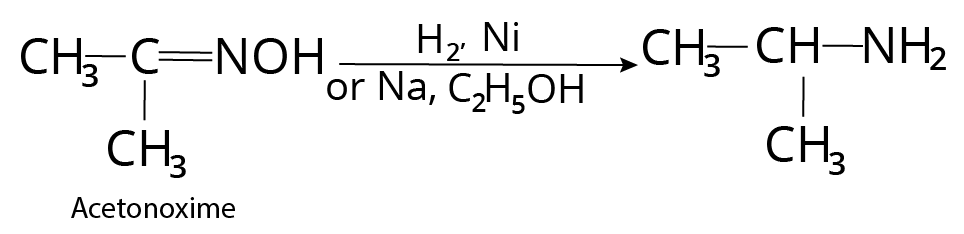
Reaction of Oxime
Example:
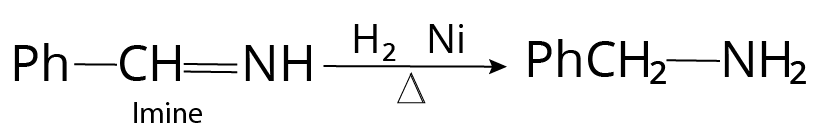
Reaction of Imine
Reduction of Nitro Compounds
Tin with hydrochloric acid or lithium aluminium hydride are widely used to convert nitroalkanes to amines. ${H_2}$ and a catalyst are also utilised in this reduction.
Reactions of Amines
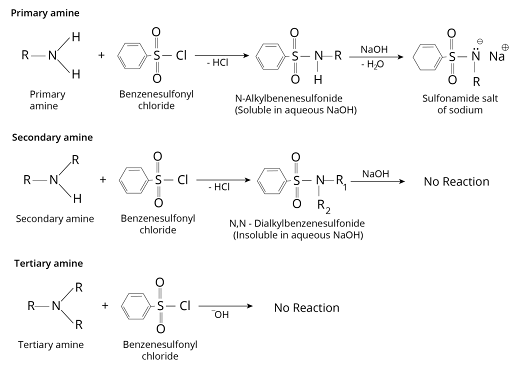
Sulphonylation – Hinsberg Test
Treatment with benzene sulphonyl chloride or p-toluene sulphonyl chloride (Hinsberg reagent).
The reaction is used to separate the amine mixture.
Primary and secondary amines react to generate matching sulphonamides in the presence of active hydrogen, while tertiary amine does not.
The main amine product, N-alkyl benzene sulphonamide, dissolves in $KOH$ and creates a water-soluble salt.
Hinsberg Test
Nitrous Acid Test
When a primary amine interacts with HNO3, alcohol is frequently the result. As an intermediate, diazonium salt is generated due to the reaction.
Secondary amine produces nitrosoamine, a yellow oily liquid.
A soluble salt forms when the tertiary amine is dissolved in cold $HN{O_2}$.
Example:
$\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{NH}_{2} \stackrel{\mathrm{HNO}_{2}}{\longrightarrow} \mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{OH}+\mathrm{N}_{2}+\mathrm{H}_{2} \mathrm{O}$
Example:
$\left(\mathrm{CH}_{3}\right)_{2} \mathrm{NH} \stackrel{\mathrm{HNO}_{2}}{\longrightarrow}\left(\mathrm{CH}_{3}\right)_{2} \mathrm{~N}-\mathrm{N}=\mathrm{O}+\mathrm{H}_{2} \mathrm{O}$
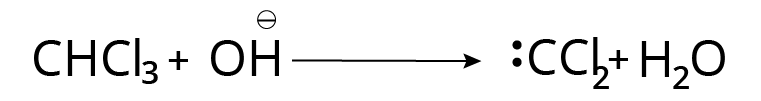
Carbylamine Reaction
When primary amine is treated with chloroform and alcoholic $KOH$, it creates pungent alkyl carbylamine vapours that are unpleasant or pungent.
The reaction is referred to as a primary amine test:
Reaction Between Chloroform and Alcohol
Treatment with $C{S_2}/HgC{l_2}$
Dithiocarbamic acid is generated when primary amine is heated with carbon sulphide which is subsequently destroyed by mercuric chloride to produce alkyl isothiocyanate. Hofmann's Mustard Oil Reaction is the name of the reaction.
Mercuric chloride does not degrade dithiocarbamic acid, which is produced by secondary amine.
Carbon sulphide has no effect on the tertiary amine.

Reaction of Carbon Sulphide with Primary, Secondary and Tertiary Amine
Acylation
When primary and secondary amines react with acid halide or anhydride, N-substituted amide is produced. In aniline, the reaction is employed to protect the ring.
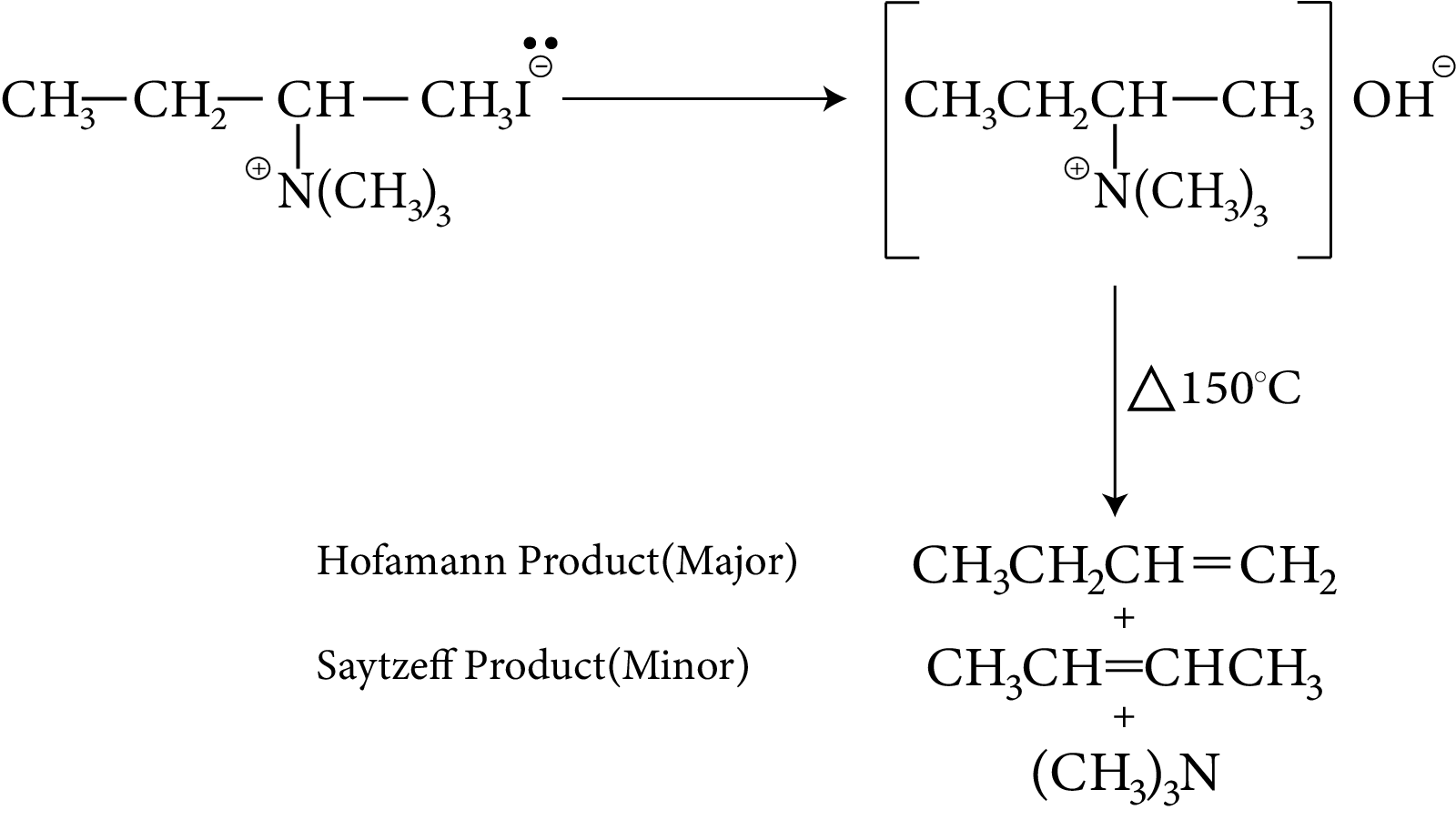
Hofmann’s Exhaustive Methylation and Elimination
Quaternary ammonium iodides are converted to hydroxides by $A{g_2}O$. The hydroxide is removed by heat, resulting in tertiary amines and alkenes. The fact that the least substituted alkene is the major product is a key factor in this process. This procedure is known as Hofmann's Elimination.
Example:
Hofmann Elimination Reaction
Metal Ions
Amines form coordination compounds with metal ions.
Preparation of Aromatic Amines
In addition to the methods for making aliphatic amines, the following methods can be employed.
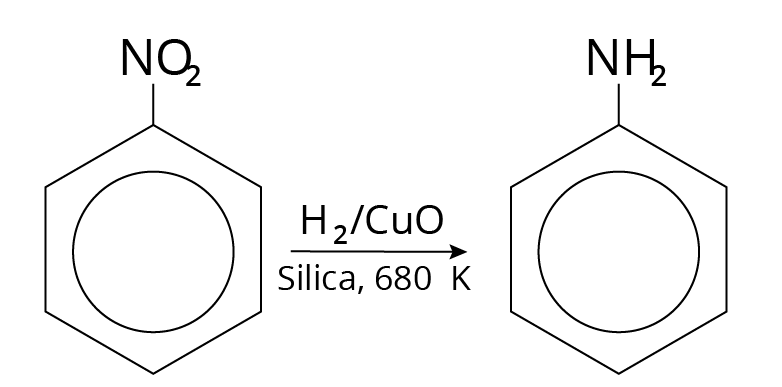
Reduction of Nitro Compounds
Vapour – Phase Reduction
The reducing agents used were: \[CuO\] on $Si{O_2}$ or $V - Pt$ Catalyst.
Example:
Vapour Phase Reduction
Catalytic Hydrogenation
The reducing agents used were ${H_2},\;Pd - C/Et - OH$
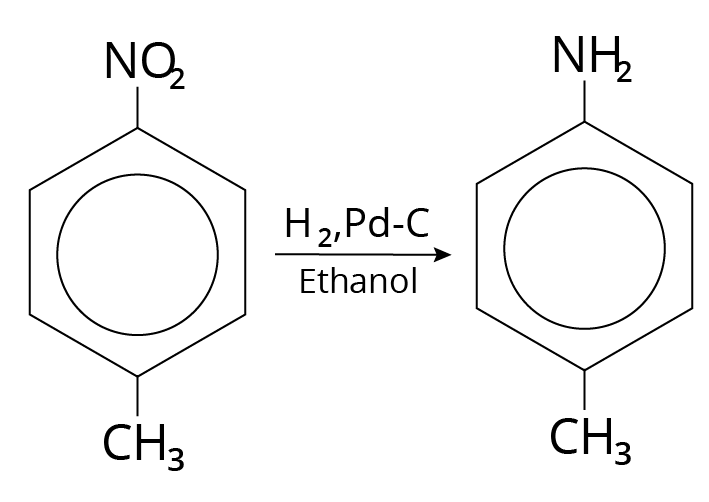
Catalytic Hydrogenation
Ammonolysis of Aryl Halides
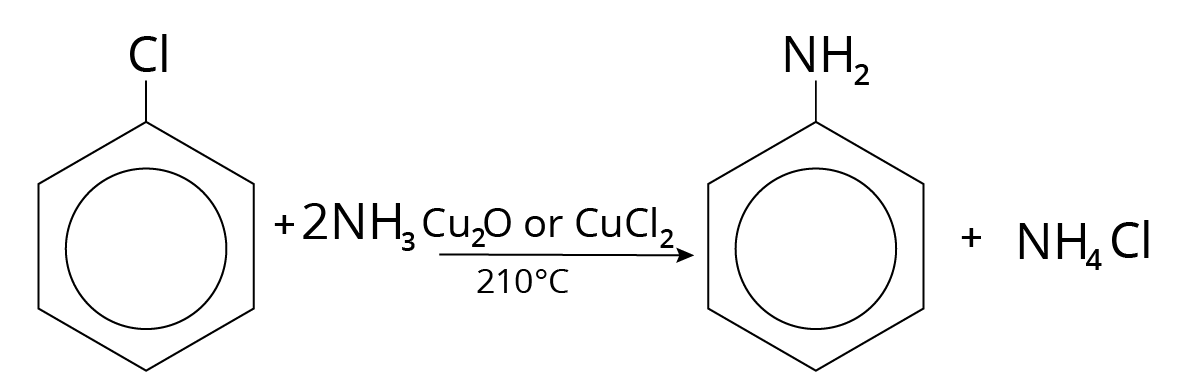
Ammonolysis of Aryl Halide
Reduction of Azo Compounds
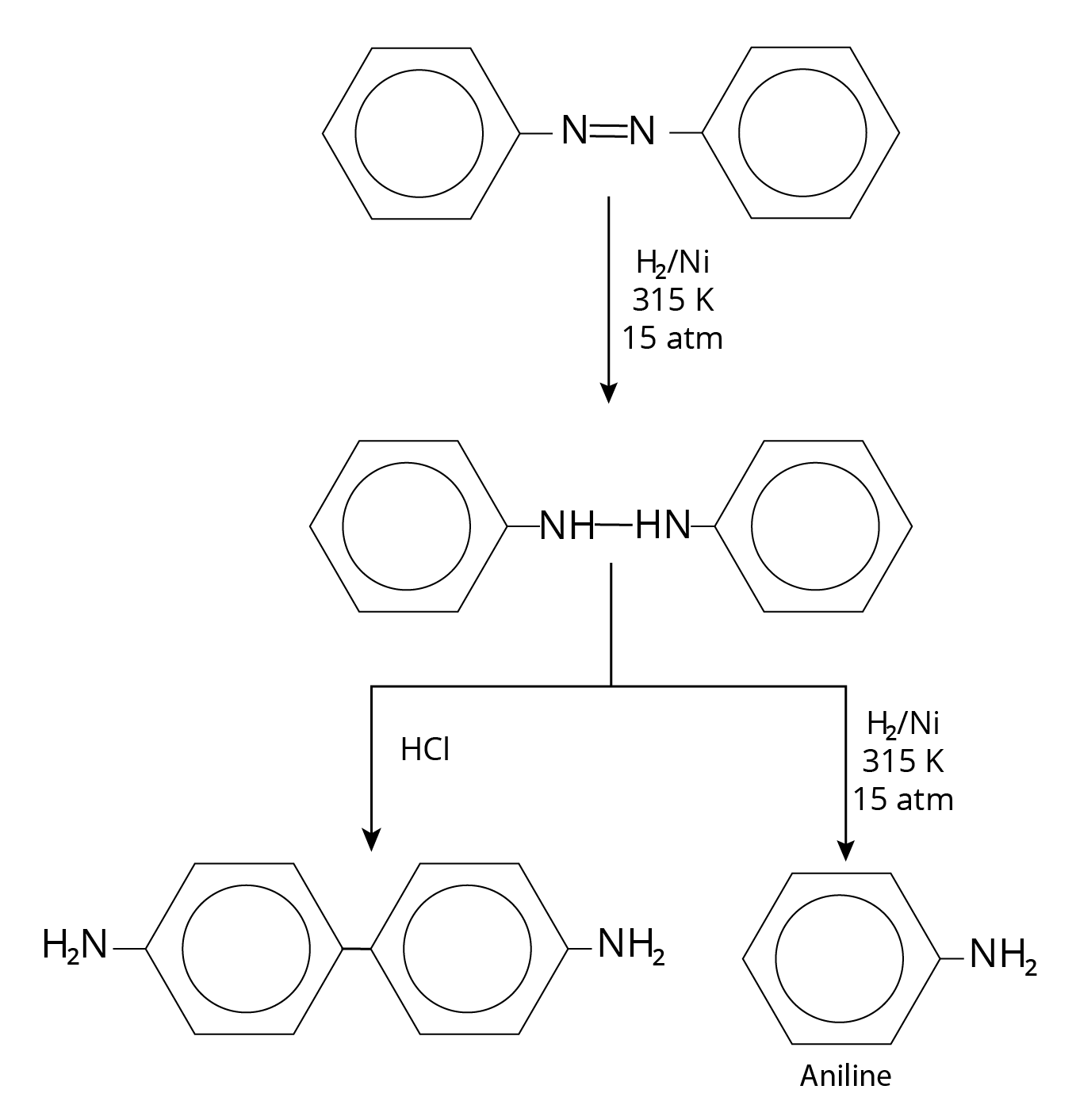
Reaction of azo Compounds
Reactions of Aromatic Amines
Diazonium Salt and Reactions
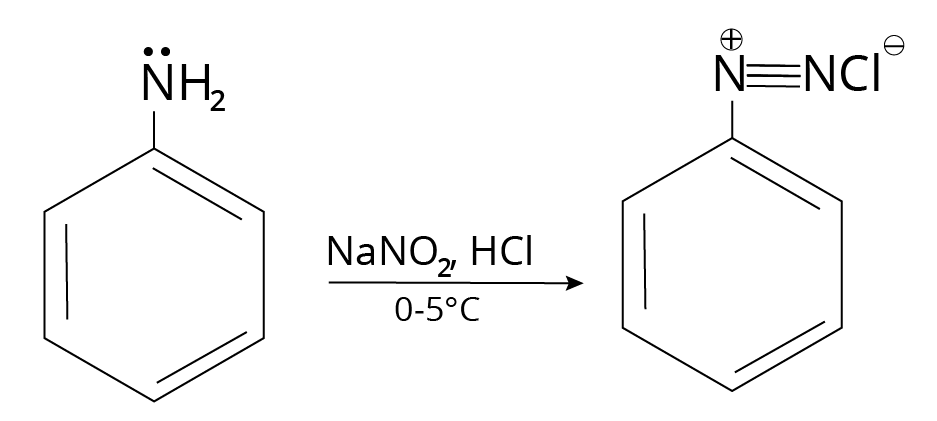
Reaction of Aromatic Amine
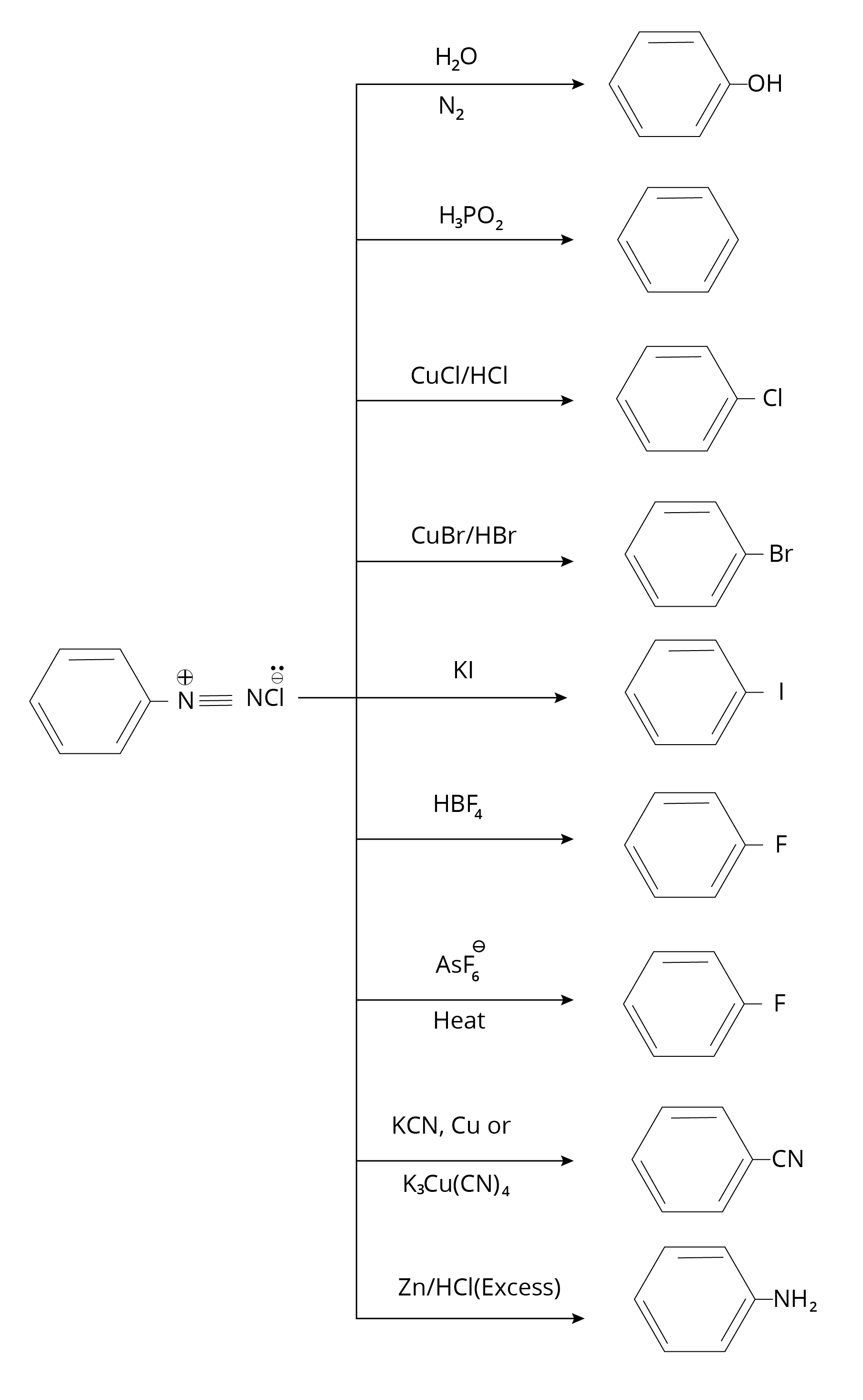
Reaction of Diazonium Salt
Oxidation
Aromatic amines can be quickly oxidised to a range of chemicals depending on the conditions. Through controlled oxidation using $N{a_2}C{r_2}{O_7}$ and ${H_2}S{O_4}$, aniline is transformed to p-benzoquinone. When a chemical is exposed to air, p-benzoquinone is produced.
Aniline black, a black dye, is produced if oxidation is not controlled.

Oxidation Reaction of Aniline
Halogenation
$(N{H_2})$ is a strong activator and ortho/para director. It produced 2,4, 6-tribromo aniline after normal bromination in ${H_2}O$.
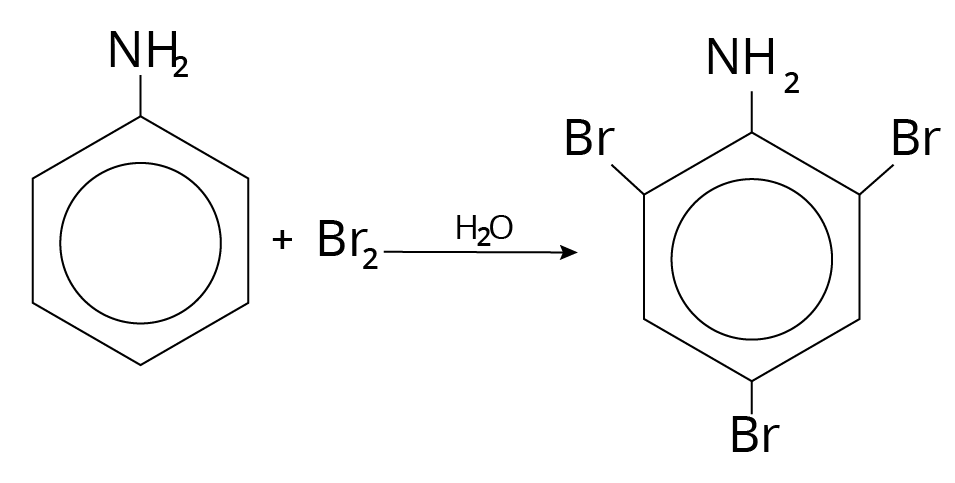
Halogenation Reaction
By lowering the activating effect of amino groups by acetylation of aniline, the $ - NHCOC{H_3}$ group can be exploited to produce ortho and para derivatives.
Nitration
Typical aniline nitration is not done because the nitrating mixture is oxidising and $N{H_2}$ is a strong activating group. Aniline is also protonated at 288 K to produce the meta-directing anilinium ion. As a result, a large number of meta derivatives are produced in addition to ortho and para derivatives.
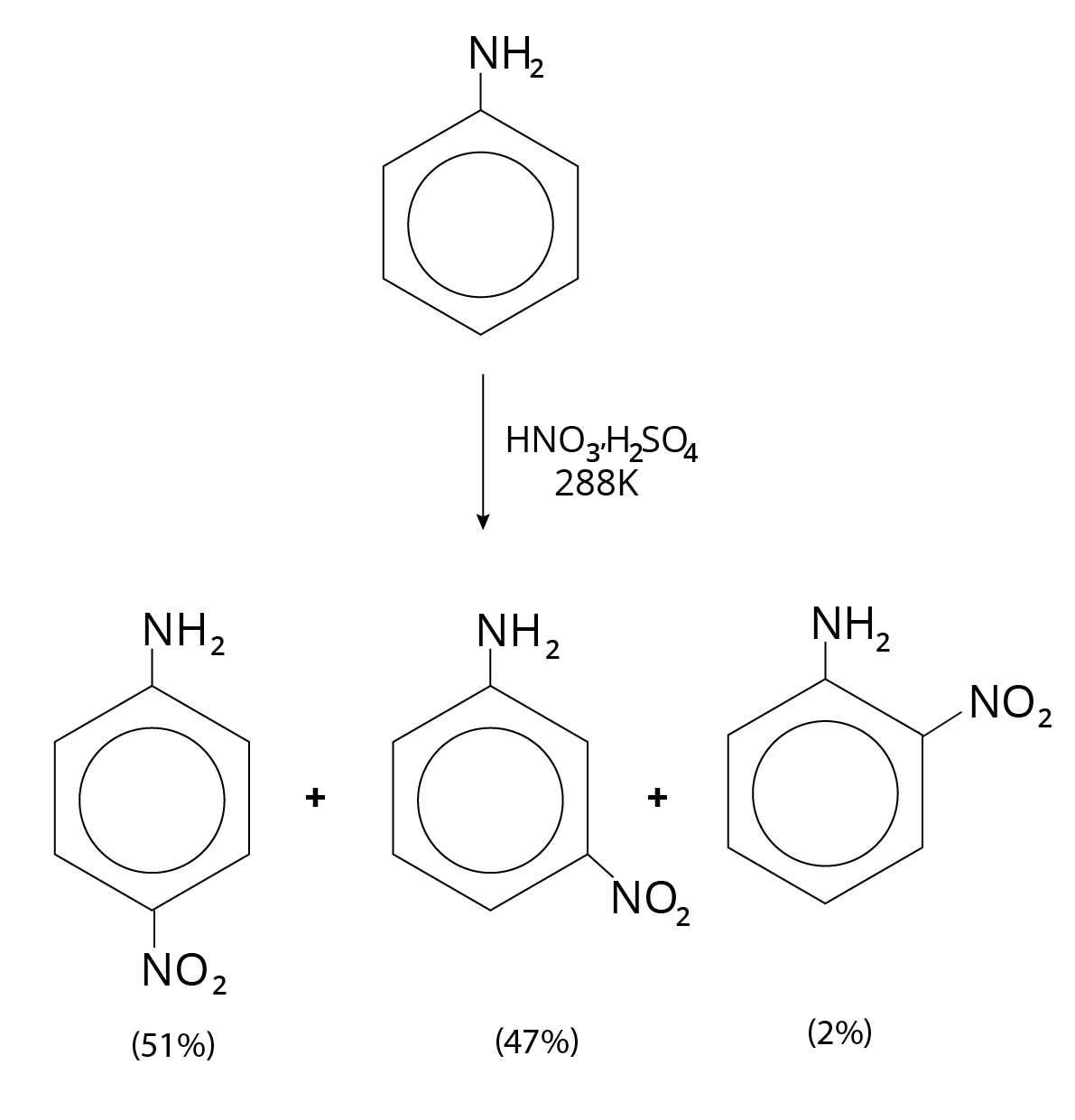
Nitration Reaction
By shielding the $ - N{H_2}$ group with an acetylation reaction with acetic anhydride, the nitration reaction can be controlled and the p-nitro derivative obtained as the main product.
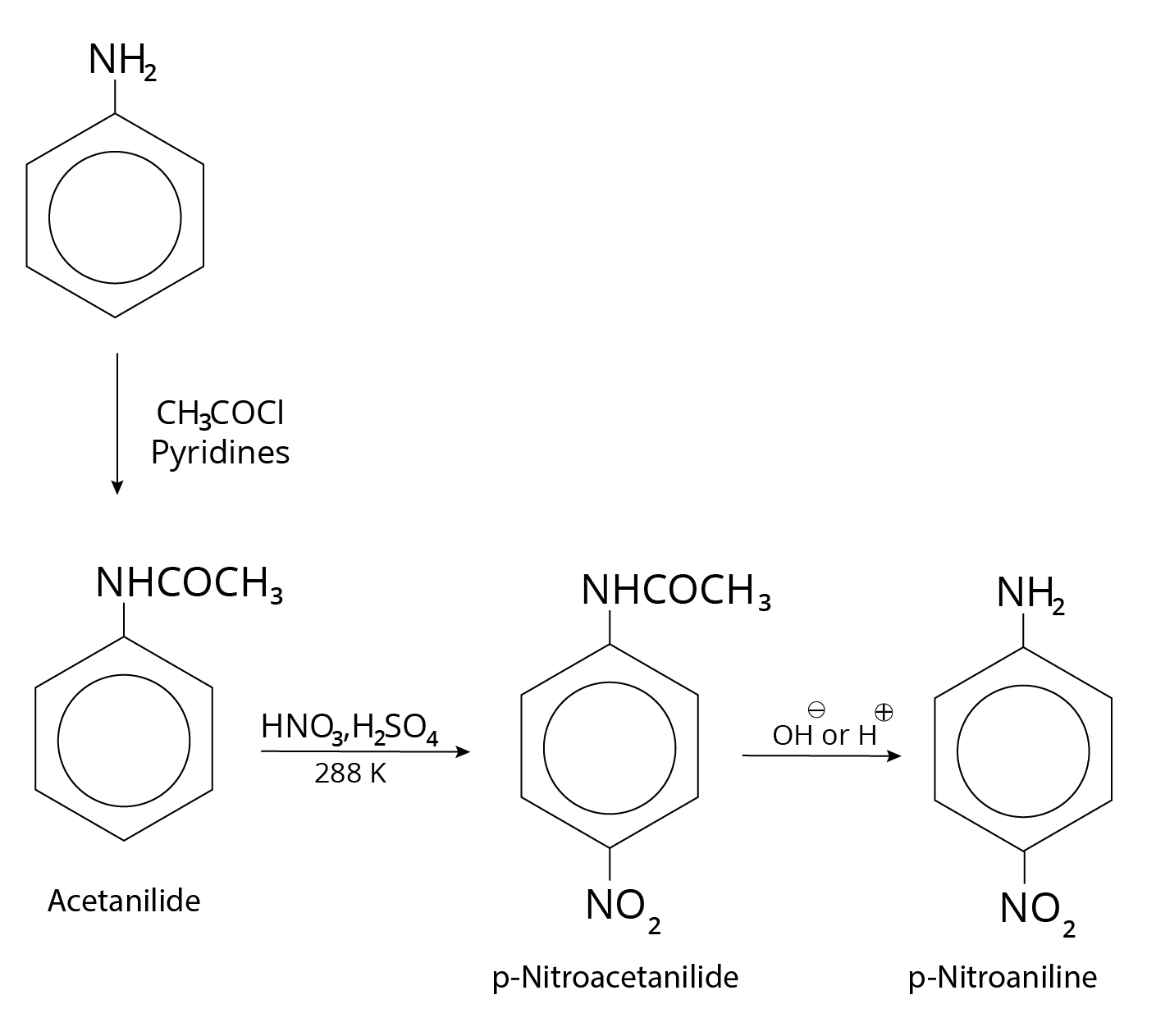
Reaction of Aniline with Pyridine
Sulphonation
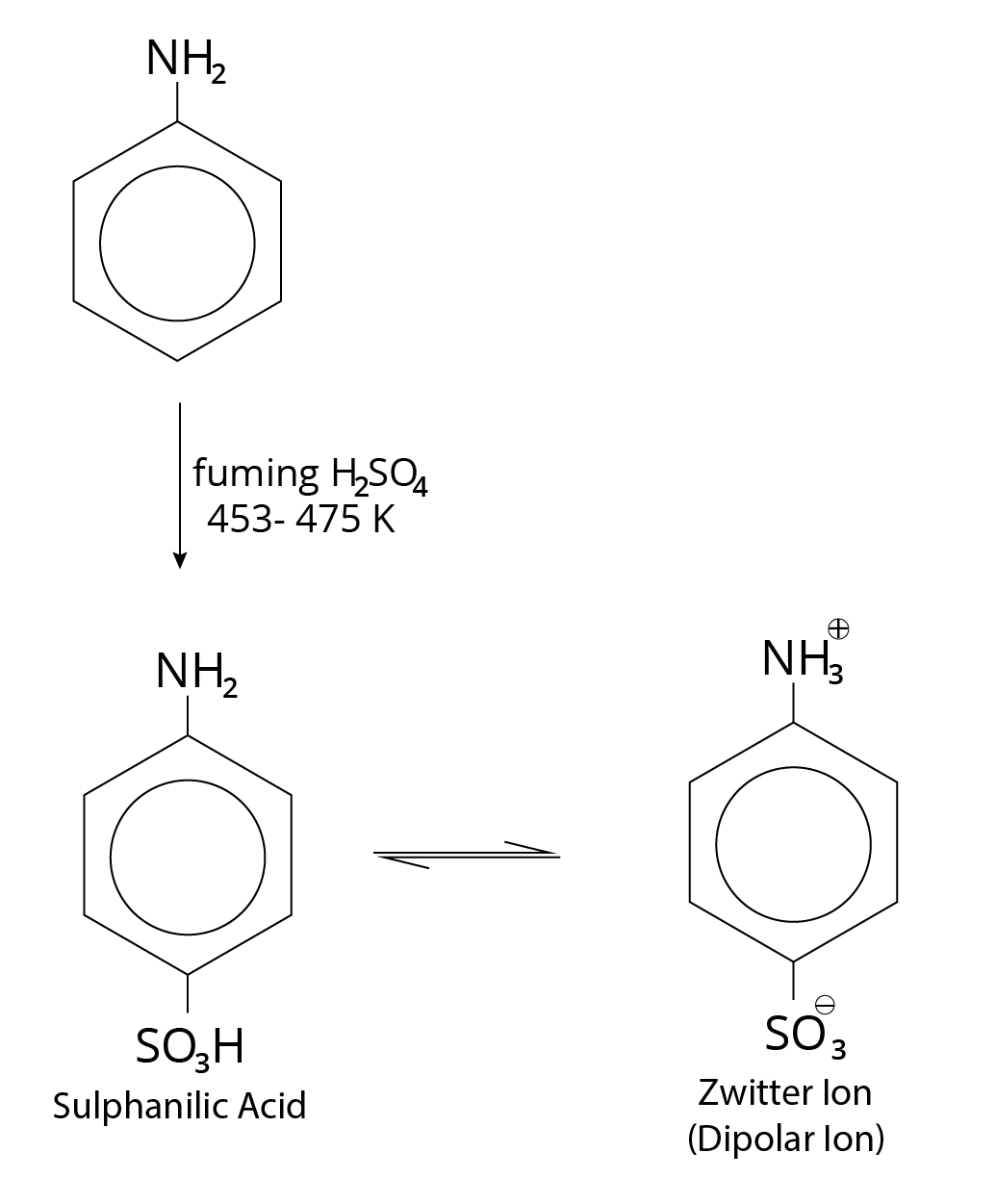
Sulphonation Reaction
Note:
The $- N{H_2}$ group attached to benzene is normally incapable of producing the zwitter ion, whereas $- S{O_3}H$ is highly acidic. Sulphanilic acid forms the zwitter ion as a result. There is no zwitter ion produced when the $- N{H_ 2}$ group is coupled to benzoic acid in any place.
Analysis of Amines
Amines is distinguished primarily by their basicity. An amine is a water-insoluble chemical that dissolves in cold dilute hydrochloric acid or a water-soluble molecule that turns litmus blue in an aqueous solution. The best approach to tell if an amine is primary, secondary, or tertiary is to use the Hinsberg test. The amine is shaken with benzenesulfonyl chloride in the presence of aqueous potassium hydroxide. Primary and secondary amines, but not tertiary amines, create substituted sulfonamides. The monosubstituted sulfonamide of a primary amine has an acidic hydrogen connected to nitrogen. By interacting with potassium hydroxide, this amide becomes a soluble salt. The disubstituted sulfonamide from a secondary amine is insoluble in the alkaline reaction mixture because it lacks an acidic hydrogen. The way an amine reacts with nitrous acid can aid in classifying it. Primary aromatic amines, in particular, have a unique behaviour: when they are treated with nitrous acid, they become diazonium salts, which when combined with beta-naphthol produce vividly coloured azo compounds.

Hinsberg Test
Solved examples:
1. The best reagent for converting-2-phenylpropanamide into 1-phenylethanamine is.
\[\begin{array}{*{20}{l}}{\left( {\rm{A}} \right){\rm{ excess~ }}{{\rm{H}}_{\rm{2}}}{\rm{/Pt}}}\\{\left( {\rm{B}} \right){\rm{ NaOH /B}}{{\rm{r}}_{\rm{2}}}}\\{\left( {\rm{C}} \right){\rm{ NaB}}{{\rm{H}}_{\rm{4}}}{\rm{/methanol}}}\\{\left( {\rm{D}} \right){\rm{ LiAl}}{{\rm{H}}_{\rm{4}}}{\rm{/ether}}}\end{array}\]
Correct Answer: Option (B)
Explanation:
The Hoffmann bromamide reaction, also known as the degradation reaction, begins with the treatment of the primary amide group with halogen (Br), and then the halogen substituted amide product is converted to primary amine with the release of carbon dioxide gas.
The reaction is shown below.
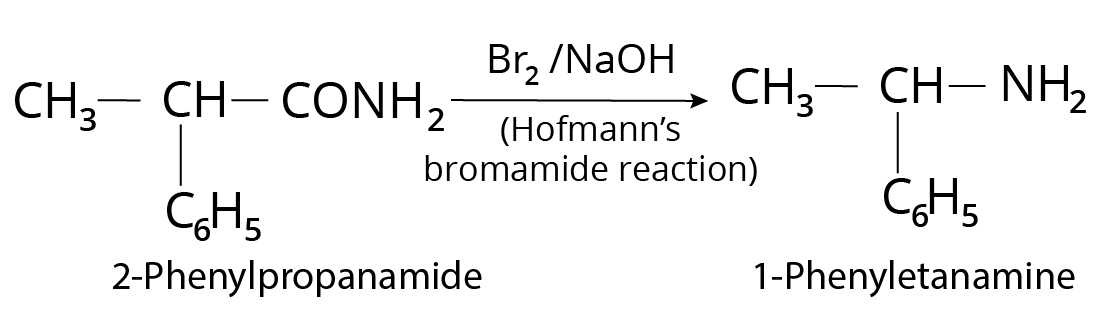
1-phenylethanamine
Thus, option (B) is correct.
2. Which of the following compounds will not undergo azo coupling reaction with benzene diazonium chloride?
(A) Aniline
(B) Phenol
(C) Anisole
(D) Nitrobenzene
Correct Answer: Option (D)
Explanation:
A weak electrophile, such as Diazonium cation, readily reacts with electron-rich compounds, such as hydroxyl groups and amine groups. They do not react with electron removing groups such as nitro groups. As a result, nitrobenzene will not undergo azo coupling with benzene diazonium chloride. Thus, option (d) is correct.
Importance of Amines in Class 12 Chemistry
This chapter is all about the organic compounds containing an amino group (-NH2) in the main chain. These compounds can be differentiated into primary, secondary, and tertiary classifications based on the position of the amino group in the carbon chain. The position of this functional group is defined by the carbon atom it is attached to.
In proceeding further in this chapter, students will learn how the amines are formed from amides, nitriles, and from alkyl compounds. There are different ways to synthesise these compounds. This chapter will explain all the reaction steps and reagents used along with the intermediate products. You will find out how the transition of these organic compounds leads to the formation of amines.
Every reaction will be explained in detailed steps so that the students can understand the mechanism of developing or substituting the amino group for a particular reactant organic compound.
This chapter is crucial for the NEET aspirants as they will understand how to achieve the desired amine from a given reactant organic compound. By understanding the concept and mechanism of the synthesis reactions, students will be able to write equations and answer fundamental questions.
To revise this chapter quickly, one can refer to the Amines notes on Vedantu prepared by our experts. These notes will provide a concise format of these reactions and other fundamental concepts of this chapter.
Benefits of Amines NEET Notes
The Amines NEET notes are prepared in a concise format so that students preparing for the exam can easily revise the chapter in an organized way. All the conceptual theories, definitions, reactions, explanations, etc., can be found in an easy-to-comprehend format in these notes.
These revision notes cover the chapter topic-wise, making it easier for students to memorize the reactions. Students can use these notes for revision purposes.
The short notes of Amines for NEET Class 12 will reduce the preparation time of the NEET aspirants and help them to cover the syllabus faster. Thus you can answer conceptual questions confidently and score well in the exams.
Download Amines Class 12 Notes Free PDF
It is time to avail the easiest revision notes for studying amines to prepare well for the upcoming NEET exam. Avail of the Amines Class 12 notes PDF download option and refer to them to revise this chapter for your NEET preparation.
FAQs on Amines Class 12 Notes NEET Chemistry (Free PDF Download)
1. What is the amino group?
The amino group (-NH2) is a functional group derived from the substitution of one constituent hydrogen of ammonia (NH3) with an organic group.
2. What happens when an amide is reduced?
When an amide is reduced using a reducing agent, it forms an amine with the carbon atom numbers in the molecule similar to the amide.
3. What is ammonia?
Ammonia (NH3) is a gas that contains a nitrogen atom attached to three hydrogen atoms to form a molecule. It is used to prepare amides in different chemical ways.
4. What is substitution reaction?
When an atom in an organic compound is substituted with a functional group, it is called a substitution reaction.
























