
Which of the following is the correct order of strength of H-bonding in the given compound?
A. ${\rm{HF}} < {\rm{N}}{{\rm{H}}_3}$
B. ${{\rm{H}}_2}{\rm{O}} > {{\rm{H}}_2}{{\rm{O}}_{\rm{2}}}$
C. ${{\rm{H}}_2}{{\rm{O}}_{\rm{2}}} > {{\rm{H}}_2}{\rm{O}}$
D. ${\rm{N}}{{\rm{H}}_3} > {{\rm{H}}_{\rm{2}}}{\rm{O}}$
Answer
233.1k+ views
Hint: Hydrogen bond is a chemical bond in which formation of a covalent link of hydrogen atoms with other electronegative atoms, such as, fluorine, nitrogen and oxygen atoms takes place in the same or another molecule.
Complete step by step answer:
We know that the extent of hydrogen bonding depends on electronegativity and the number of hydrogen atoms available for bonding. Among Fluorine, nitrogen and oxygen atoms, increasing order of electronegativities is ${\rm{N}} < {\rm{O}} < {\rm{F}}$.
> Between ammonia $\left( {{\rm{N}}{{\rm{H}}_3}} \right)$ and hydrogen fluoride $\left( {{\rm{HF}}} \right)$, HF has more H-bonding than ${\rm{N}}{{\rm{H}}_3}$ because in ammonia, only one lone pair present which cannot satisfy all hydrogen also fluorine is more electronegative than nitrogen. So, option A is incorrect.
> Between water $\left( {{{\rm{H}}_2}{\rm{O}}} \right)$and hydrogen peroxide $\left( {{{\rm{H}}_2}{{\rm{O}}_{\rm{2}}}} \right)$, hydrogen peroxide has more H-bonding because of presence of one more oxygen that water. So, option B is incorrect and option C is correct.
> Between ammonia $\left( {{\rm{N}}{{\rm{H}}_3}} \right)$ and water $\left( {{{\rm{H}}_2}{\rm{O}}} \right)$, water has more H-bonding than ammonia $\left( {{\rm{N}}{{\rm{H}}_3}} \right)$ because of more number of hydrogen bond and also electronegativity of Oxygen is more than Nitrogen .
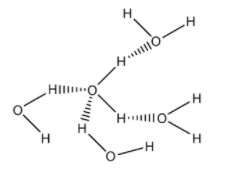
In case of water, it can form four hydrogen bonds with four other water molecules. But in case of ammonia, only one lone pair is present, which can form only one hydrogen bond with one ammonia molecule.
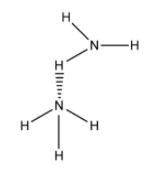
So, water molecules have more extent of hydrogen bonding. So, option D is incorrect.
Note: The extent of hydrogen bonding is in the order ${\rm{H}} - {\rm{F}} > {\rm{H}} - {\rm{O}} > {\rm{H}} - {\rm{N}}$, that means, extent of hydrogen bonding is more in case of HF than water $\left( {{{\rm{H}}_2}{\rm{O}}} \right)$, that is, ${\rm{HF}} > {{\rm{H}}_{\rm{2}}}{\rm{O}}$ . But we also have to consider the effect of the number of hydrogen bonding in the molecule. Water can form four H-bonds with four other hydrogen atoms but HF can form only one H-bond. So, HF has less effect of H-bonding than ${{\rm{H}}_2}{\rm{O}}$.
Complete step by step answer:
We know that the extent of hydrogen bonding depends on electronegativity and the number of hydrogen atoms available for bonding. Among Fluorine, nitrogen and oxygen atoms, increasing order of electronegativities is ${\rm{N}} < {\rm{O}} < {\rm{F}}$.
> Between ammonia $\left( {{\rm{N}}{{\rm{H}}_3}} \right)$ and hydrogen fluoride $\left( {{\rm{HF}}} \right)$, HF has more H-bonding than ${\rm{N}}{{\rm{H}}_3}$ because in ammonia, only one lone pair present which cannot satisfy all hydrogen also fluorine is more electronegative than nitrogen. So, option A is incorrect.
> Between water $\left( {{{\rm{H}}_2}{\rm{O}}} \right)$and hydrogen peroxide $\left( {{{\rm{H}}_2}{{\rm{O}}_{\rm{2}}}} \right)$, hydrogen peroxide has more H-bonding because of presence of one more oxygen that water. So, option B is incorrect and option C is correct.
> Between ammonia $\left( {{\rm{N}}{{\rm{H}}_3}} \right)$ and water $\left( {{{\rm{H}}_2}{\rm{O}}} \right)$, water has more H-bonding than ammonia $\left( {{\rm{N}}{{\rm{H}}_3}} \right)$ because of more number of hydrogen bond and also electronegativity of Oxygen is more than Nitrogen .

In case of water, it can form four hydrogen bonds with four other water molecules. But in case of ammonia, only one lone pair is present, which can form only one hydrogen bond with one ammonia molecule.

So, water molecules have more extent of hydrogen bonding. So, option D is incorrect.
Note: The extent of hydrogen bonding is in the order ${\rm{H}} - {\rm{F}} > {\rm{H}} - {\rm{O}} > {\rm{H}} - {\rm{N}}$, that means, extent of hydrogen bonding is more in case of HF than water $\left( {{{\rm{H}}_2}{\rm{O}}} \right)$, that is, ${\rm{HF}} > {{\rm{H}}_{\rm{2}}}{\rm{O}}$ . But we also have to consider the effect of the number of hydrogen bonding in the molecule. Water can form four H-bonds with four other hydrogen atoms but HF can form only one H-bond. So, HF has less effect of H-bonding than ${{\rm{H}}_2}{\rm{O}}$.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




