
In \[Ph-CH(OH)C{{H}_{3}}\xrightarrow{SOC{{l}_{2}}}Ph-CH(Cl)C{{H}_{3}}+S{{O}_{2}}+HCl\]
Which of the following acts as a leaving group?
A. \[O{{H}^{-}}\]
B. \[C{{l}^{-}}\]
C. \[S{{O}_{2}}\]
D. 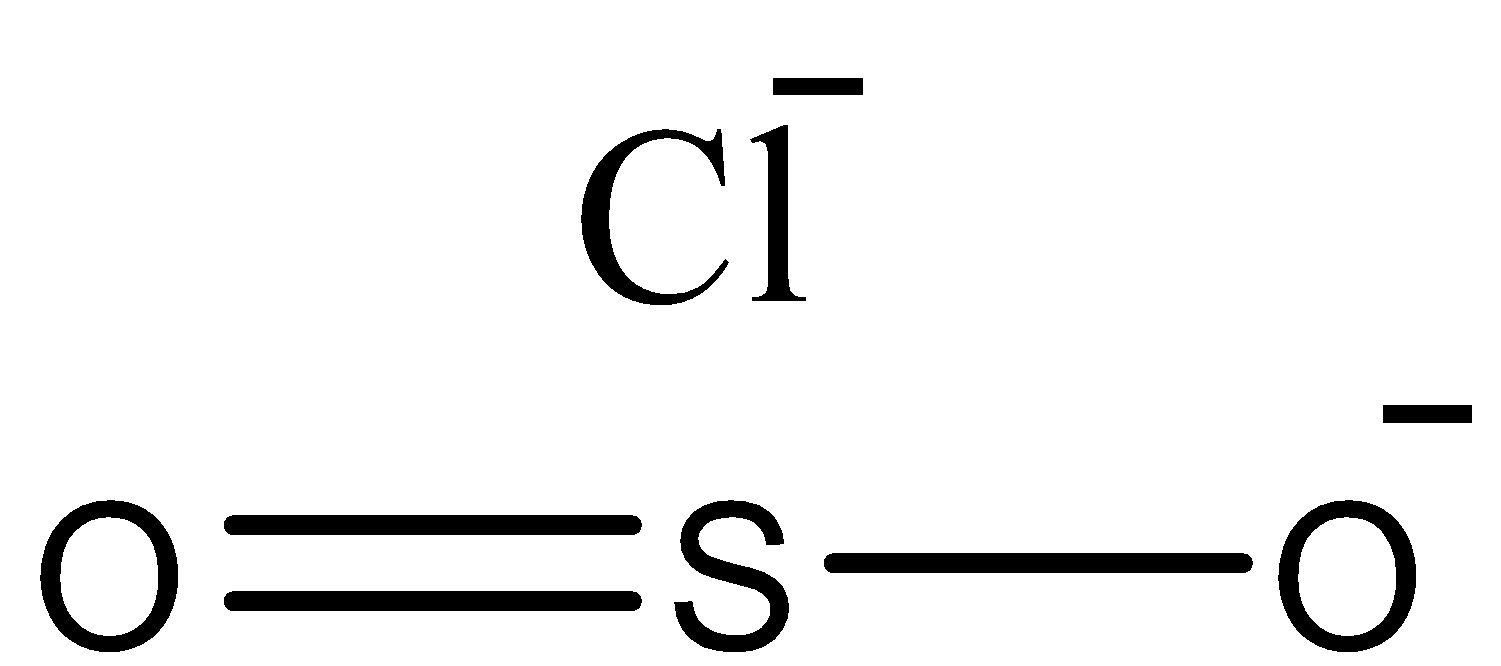
Answer
232.8k+ views
Hint: Alcohols react with thionyl chloride and form their chloro derivatives (alkyl or aryl halides) as the products. This reaction is called chlorination. Chlorination is a best example for substitution reactions.
Complete step by step answer:
* In the given reaction aryl halide is reacting with thionyl chloride and forming its halide derivative and Sulphur dioxide and hydrochloric acid as by products.
* In the question it mentioned that we have found the leaving group in the reaction.
* To know about the leaving group we should write the mechanism of the given reaction.
The mechanism of the given reaction is as follows.
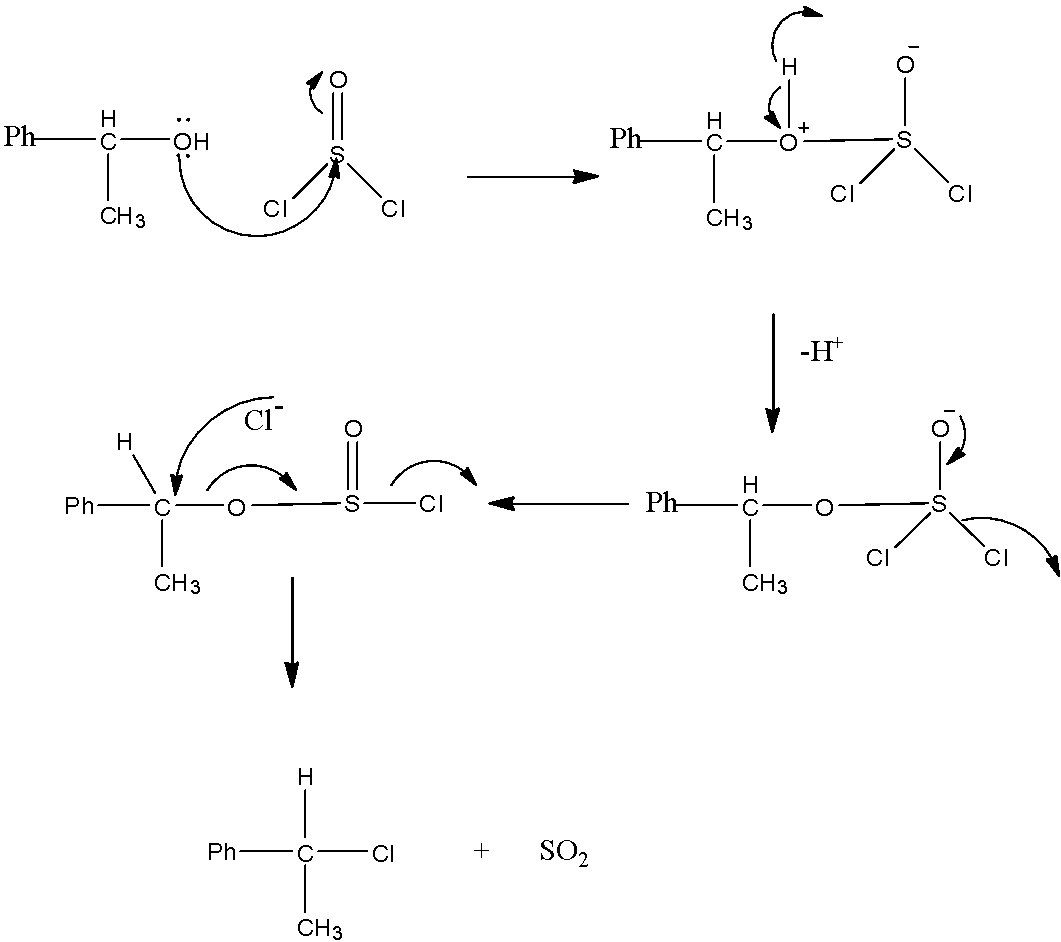
* In the first step, a lone pair of electrons on oxygen on alcohol attacks on the electron deficient Sulphur atom.
* Later to stabilize the hydrogen atom of alcohol comes out as a hydrogen ion, in continuation the negative charge on the oxygen attached to Sulphur is getting stabilized by donating electrons to Sulphur in the process chloride ion comes out.
* The liberated chloride ion attacks on the carbon which is attached to Sulphur through oxygen.
* At that time in the process of stabilization Sulphur dioxide was liberated as a by-product.
* In the given reaction the liberated product is Sulphur dioxide.
So, the correct option is C.
Note: All of us think that in the given reaction the hydroxyl group is converting into halogen in the product. So, the liberated product is the hydroxide group (\[O{{H}^{-}}\]). But through mechanism only we can say which group is going to substitute in the reaction.
Complete step by step answer:
* In the given reaction aryl halide is reacting with thionyl chloride and forming its halide derivative and Sulphur dioxide and hydrochloric acid as by products.
\[Ph-CH(OH)C{{H}_{3}}\xrightarrow{SOC{{l}_{2}}}Ph-CH(Cl)C{{H}_{3}}+S{{O}_{2}}+HCl\]
* In the question it mentioned that we have found the leaving group in the reaction.
* To know about the leaving group we should write the mechanism of the given reaction.
The mechanism of the given reaction is as follows.

* In the first step, a lone pair of electrons on oxygen on alcohol attacks on the electron deficient Sulphur atom.
* Later to stabilize the hydrogen atom of alcohol comes out as a hydrogen ion, in continuation the negative charge on the oxygen attached to Sulphur is getting stabilized by donating electrons to Sulphur in the process chloride ion comes out.
* The liberated chloride ion attacks on the carbon which is attached to Sulphur through oxygen.
* At that time in the process of stabilization Sulphur dioxide was liberated as a by-product.
* In the given reaction the liberated product is Sulphur dioxide.
So, the correct option is C.
Note: All of us think that in the given reaction the hydroxyl group is converting into halogen in the product. So, the liberated product is the hydroxide group (\[O{{H}^{-}}\]). But through mechanism only we can say which group is going to substitute in the reaction.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




