Acids, Bases and Salts Solutions for Class 10 Science ICSE Board (Concise - Selina Publishers)
Free download of step by step solutions for class 10 Science (Chemistry) Chapter 3 - Acids, Bases and Salts of ICSE Board (Concise - Selina Publishers). All exercise questions are solved & explained by an expert teacher and as per ICSE board guidelines.
Acids, Bases, and Salts deals with different reactions and students learn about different properties of acids, bases, and salts in different conditions. Students learn about real-life applications of acids, bases, and salts. Students can understand the topics and equations by accessing free Concise Acids, Bases and Salts Solutions for Class 10 Science ICSE Board.
Access ICSE Selina Solutions for Grade 10 Chemistry Chapter 03 – Acids, Bases and Salts
Intext – Question – 1
1. (a) What do you understand about the term acid?
Ans: Acids are defined as compounds which contain one or more hydrogen atoms, and when dissolved in water, they produce hydronium ions (H3O+), the only positively charged ions.
(b) Name the positive ion formed when an acid is dissolved in water.
Ans: Hydronium ion is the positive ion formed when an acid is dissolved in water.
(c) Draw the structure of this ion.
Ans:
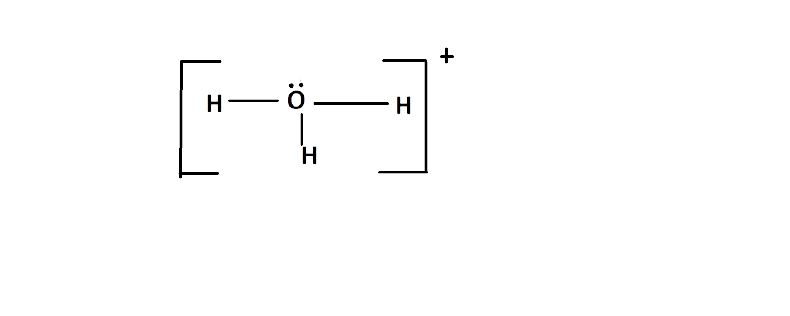
2. Write ionization of sulphuric acid showing the formation of hydronium ions.
Ans: H2SO4 → H+ + HSO4-
H+ + H2O → H3O+
3. Water is never added to acid in order to dilute it. why?
Ans: If water is added to a concentrated acid, the heat generated causes the mixture to splash out and cause severe burns. Thus, water is never added to acid in order to dilute it.
4. Define the term basicity of an acid. Give the basicity of: nitric acid, sulphuric acid, and phosphoric acid.
Ans: Basicity: The basicity of an acid is defined as the number of hydronium ions (H3O+) that can be produced by the ionization of one molecule of that acid in aqueous solution.
The basicity of following compounds are: Nitric acid: Basicity = 1
Sulphuric acid: Basicity =2
Phosphoric acid: Basicity =3
5. Give two examples of each of the following:
a. Oxyacids
Ans: HNO3, H2SO4
b. Hydracid
Ans: HCl, HBr
c. Monobasic acid
Ans: HCl, HBr
d. Dibasic acid
Ans: H2SO4, H2CO3
e. Tribasic acid
Ans: H3PO4, C6H8O7
6. Name the:
a. Acidic anhydride of the following acids.
i. Sulphurous acid
Ans: SO2
ii. Nitric acid
Ans: N2O5
(iii) Phosphoric acid
Ans: P2O5
(iv) Carbonic acid
Ans: CO2
b. Acids present in vinegar, grapes, and lemon.
Ans: The acid present in Vinegar is Acetic acid, in Grapes is Tartaric acid, and in Lemon is Citric acid.
7. What do you understand by the statement acetic acid is monobasic acid?
Ans: Acetic acid is a monobasic acid which on ionization in water produces one hydronium ion per molecule of the acid.
8. Give a balanced equation for:
(i) Reaction of nitrogen dioxide with water
Ans: 2NO2 + H2O ⟶ HNO2 + HNO3
(ii) Preparation of non-volatile acid from volatile acid.
Ans: Sulphuric acid is a non-volatile acid, therefore, used in the preparation of volatile acids like hydrochloric acid and nitric acid. The non-volatile acid (sulphuric acid) is prepared by using volatile acid like nitric acid. Let’s see the reaction below for this preparation:
S + HNO3 ⟶ 6NO2 + 2H2O + H2SO4
9. What do you understand about the strength of an acid? On which factor does the strength of an acid depend?
Ans: The strength of an acid is the extent to which the acid ionizes or dissociates in water. The strength of an acid depends on the degree of ionization and concentration of hydronium ions [H3O+] produced by that acid in aqueous solution.
10. Explain the following:
a. Carbonic acid gives an acid salt but hydrochloric does not.
Ans: Carbonic acid is a dibasic acid with two replaceable hydrogen ions; therefore it forms one acid salt or one normal salt. Hydrochloric acid is a monobasic acid with one replaceable hydrogen ion and so forms only one normal salt.
b. Dil. HCl is stronger than highly concentrated acetic acid.
Ans: Strength of an acid is the measure of concentration of hydronium ions it produces in its aqueous solution. Dil. HCl produces high concentration of hydronium ions compared to that of concentrated acetic acid. Thus, dil. HCl is a stronger acid than highly concentrated acetic acid.
Exercise – 3A
1. What do you understand about alkali? Give two examples of (a). Strong alkali and (b) weak alkali.
Ans: An alkali is a basic hydroxide which when dissolved in water produces hydroxyl ions (OH-) as the only negatively charged ions.
(a) Strong alkalis: Sodium hydroxide, Potassium hydroxide
(b) Weak alkalis: Calcium hydroxide, Ammonium hydroxide
2. What is the difference between?
a. An alkali and base.
Ans: An alkali and a base:
Alkali | Base |
Alkalis are soluble in water. | Bases may be or may not be soluble in water. |
All alkalis are bases | All bases are not alkalis. |
b. The chemical nature of an aqueous solution HCl and an aqueous solution of NH3.
Ans:
Aqueous solution of HCl | Aqueous solution of NH3 |
It is acidic in nature. | It is basic in nature. |
It will turn blue litmus to red. | It will turn red litmus to blue. |
3. Name the ions furnished by:
a. Bases in the solution.
Ans: Bases are the compounds which when dissolved in water produce hydroxyl ions.
b. An acid.
Ans: Acids when dissolved in water produce hydronium ions.
4. Give one example in each case:
a. A basic oxide which is soluble in water.
Ans: The oxide of sodium (Na), potassium (K), and ammonium (NH3) are soluble in water.
b. A hydroxide which is highly soluble in water.
Ans: Sodium hydroxide is a base that is highly soluble in water.
c. A basic oxide which is insoluble in water.
Ans: Copper oxide is a basic oxide that is insoluble in water due to the presence of tightly bound oxygen atoms, by high electrostatic forces, in copper oxide.
d. A hydroxide which is insoluble in water.
Ans: Beryllium hydroxide is completely insoluble in water.
e. A weak mineral acid.
Ans: Boric acid is the weak mineral acid.
f. A base which is not an alkali.
Ans: copper oxide is a base which is not an alkali.
g. An oxide which is a base.
Ans: Sodium oxide is a base.
h. A hydrogen containing compound which is not an acid.
Ans: Methane is a hydrogen containing compound which is not an acid.
i. A base which does not contain a metal ion.
Ans: Ammonia is a base that does not contain metal ion
5. You have been provided with three test tubes. One of them contains distilled water and the other two have an acidic solution and a basic solution respectively. If you are given only red litmus paper, how will you identify the contents of each test tube?
Ans: The test tube containing distilled water does not affect the red litmus paper. The test tube containing acidic solution does not change the red litmus paper. But the test tube containing the basic solution turns red litmus paper blue.
6. HCl, HNO3, C2H5OH, C6H12O6 all contain H atoms but only HCl and HNO3 show acidic character. why?
Ans: It is because HCl and HNO3 ionize in aqueous solution whereas ethanol and glucose do not ionize in aqueous solution.
7. a. Dry HCl gas does not change the colour of the dry litmus paper. why?
Ans: It is because HCl ionizes only in aqueous solution.
b. Is PbO2 a base or not? comment
Ans: Lead oxide is not a base because when it reacts with acid it forms chlorine along with salt and water. Thus, it is excluded from the class bases.
PbO2 + 4HCl → PbCl2 + Cl2 + 2H2O
c. Do basic solutions also have H+ (aq)? Explain why they are basic by taking an example.
Ans: Yes, basic solutions also have H+ (aq) ions. Basic solutions have lower concentration of H+ (aq) in comparison to concentration of OH− (aq) ions.
8. How would you obtain:
a. A base from another base.
Ans: We can obtain a base from another base by double decomposition. The aqueous solution of salts with base precipitates the respective metallic hydroxide.
FeCl3 + 3NaOH ⟶ Fe(OH)3 + 3NaCl
b. An alkali from a base.
Ans: (Na)2CO3 + Ca(OH)2 ⟶ 2NaOH + CaCO3 on heating.
(c) Salt from another salt.
Ans: NH4CI + NaOH ⟶ NaCI + H2O + NH3
9. Write a balanced equation to satisfy each statement.
a. Acid + Active metal ⟶ salt + hydrogen
Ans: Mg +2HCl ⟶ MgCl2 + H2
b. Acid + Base ⟶ salt + water
Ans: HCl + NaOH ⟶ NaCl + H2O
c. Acid + carbonate/bicarbonate ⟶ salt + water + carbon dioxide
Ans: CaCO3 +2HCl ⟶ CaCl2 +H2O + CO2
d. Acid + sulfite/bisulfite ⟶ salt + water + sulphur dioxide
Ans: CaSO3 + 2HCl ⟶ CaCl2 + H2O+ SO2
e. Acid + sulphide ⟶ salt + hydrogen sulphide
Ans: ZnS + 2HCl ⟶ ZnCl2 + H2S
10. The skin has and needs natural oils. Why is it advisable to wear gloves while working with strong alkali?
Ans: As we know, alkalis react with oil to form soap. As our skin contains oil so when we touch strong alkalis, a reaction takes place and a soapy solution is formed. Hence we should wear gloves.
11. Complete the table:
Indicator | Neutral | Acidic | Alkaline |
Litmus | purple | …………... | …………... |
Phenolphthalein | colourless | …………... | …………... |
Ans:
Indicator | Neutral | Acidic | Alkaline |
Litmus | purple | Blue to red | Red to blue |
Phenolphthalein | colourless | colourless | Pink |
12. What do you understand by pH value? Two solutions x and y have pH values of 4 and 10 respectively. Which one of these two will give a pink colour with a phenolphthalein indicator?
Ans: The pH of water is a measurement of how acidic or basic it is. The range is 0 to 14, with 7 being the neutral value. The solution Y will give a pink colour with phenolphthalein.
13. You are supplied with five solutions: A, B, C, D and E with pH values as follows:
A= 1.8, B= 7, C= 8.5, D= 13, and E= 5
Classify these solutions as neutral, slightly or strongly acidic and slightly or strongly alkaline.
Which solution would be most likely to liberate hydrogen with:
(a) Magnesium powder
(b) Powdered zinc metal. Give a word equation for each reaction.
Ans: Solution A is strongly acidic, solution B is neutral, solution C is weakly alkaline, solution D is Strongly alkaline, solution E is Weakly acidic.
(a) Solution A (acidic solution) will react with Mg powder.
(b) Solution A (acidic solution) will react with Zn powder.
Zn + Mg salt ⟶ H2 + Zn salt
14. Distinguish between:
a. A common acid base indicator and a universal indicator
Ans: Acid base indicator like litmus (common acid base indicator) tells us only whether a given substance is an acid or a base. Universal indicator gives an idea as to how acidic or basic a substance is and universal indicator gives different colours with solutions of different pH values.
b. Acidity of bases and basicity of acid.
Ans: The number of hydroxyl ions that can be produced per molecule of a base in aqueous solution is known as acidity of bases.
Acid basicity is defined as the number of hydronium ions created by ionising one molecule of the acid in aqueous solution.
c. Acid and alkali (other than indicator)
Ans: An acid is a substance that produces hydrogen ions, H+(aq), when dissolved in water.
The higher the concentration of hydrogen ions in the solution, the lower the pH. An alkali is a substance that produces hydroxide ions, OH-(aq), when dissolved in water.
15. What should be added to
a. To increase the pH value.
Ans: A base should be added to increase the pH value of the solution.
b. Decrease the pH value of a neutral solution.
Ans: An acid should be added to decrease the pH value of the neutral solution.
16. How does tooth enamel get damaged? What should be done to prevent it?
Ans: Tooth decay begins when the pH falls below 5.5. Enamel on our teeth is the toughest substance in our bodies, and it corrodes. Brush, floss, and rinse with a fluoride and antiseptic mouthwash on a daily basis to prevent enamel loss and maintain teeth healthy.
17. When you use a universal indicator, you see that solutions of different acids produce different colours. Indeed, solutions of the same acid with different concentrations will also give different colours. Why?
Ans: Universal indicator gives an idea as to how acidic or basic a substance is an universal indicator gives different colours with solutions of different pH values.
18. (a) A solution has a pH of 7. Explain how you would increase (i) increase its pH; (ii) decrease its pH.
Ans: A base should be added to increase the pH value of the solution. An acid should be added to decrease the pH value of the neutral solution.
(b). If a solution changes the colour of litmus from red to blue, what can you say about its pH?
Ans: In a basic medium red litmus changes into blue. For a basic medium pH should be more than 7.
(c) What can you say about the pH of a solution that liberates carbon dioxide from sodium carbonate?
Ans: The solution that releases carbon dioxide from sodium carbonate has a pH of less than 7, making it acidic.
19. Solution P has a pH of 13, solution Q has a pH of 6 and solution R has a pH of 2. Which
(a) Will liberate ammonia from ammonium sulphate on heating?
Ans: A solution with highest pH will release ammonia from ammonium sulphate. Therefore, the answer will be P.
Ammonium salt + alkali → Salt + water + ammonia
(b) Is it a strong acid?
Ans: The solution with lowest pH will be strong acid. So, R will be a strong acid.
(c) Contains molecules as well as ions?
Ans: Solution Q with pH 6, will contain molecules as well as ions. As, this is the weak acid, therefore, will not be able to completely dissociate into ions.
20. M is an element in the form of powder. M burns in oxygen and the product obtained is soluble in water. The solution is tested with litmus. Write down only the word which will correctly complete each of the following sentences.
(a) If M is metal, then the litmus will turn?
Ans: Litmus will turn blue.
(b) If M is non-metal, then litmus will turn?
Ans: Litmus will turn red.
(c) If M is reactive metal then _______ will be evolved when M reacts with dilute sulphuric acid.
Ans: Hydrogen gas will be evolved.
(d) If M is metal, it will form _______ oxide, which will form _______ Solution with water.
Ans: If M is metal, it will form basic oxide, which will form basic Solution with water.
(e) If M is a non-metal, it will not conduct electricity in the form of _______
Ans: It will not conduct electricity in the form of graphite.
Exercise – 3B
1. Define the following and give two examples in each case:
(a) A normal salt
Ans: Normal salts are the salts formed by the complete replacement of the ionizable hydrogen atoms of an acid by a metallic or an ammonium ion. Examples: Na2SO4, NaCl
(b) An acid salt
Ans: Acid salts are formed by the partial replacement of the ionizable hydrogen atoms of a polybasic acid by a metal or an ammonium ion. Examples: NaHSO4, Na2HPO4
(c) A basic salt
Ans: Alkali salts, also known as basic salts, are salts formed when a strong base and a weak acid are not completely neutralised. Examples: CaCO3
2. Answer the following questions related to salts and their preparations:
(a) What is a ‘salt’?
Ans: Salt is a compound formed by the partial or total replacement of the ionizable hydrogen atoms of an acid by a metallic ion or an ammonium ion.
(b) What kind of salt is prepared by precipitation?
Ans: An insoluble salt can be prepared by precipitation.
(c) Name a salt prepared by direct combination. Write an equation for the reaction that takes place in preparing the salt you have named.
Ans: A salt prepared by direct combination is Iron (III) chloride.
Reaction:
2Fe +3Cl2 → 2FeCl3
(d) Name the procedure used to prepare sodium salt such as sodium sulphate.
Ans: The name of the procedure used to prepare a sodium salt such as sodium sulphate is Neutralization of acid with base.
3. Explain the following methods with examples.
(a) Direct combination
Ans: A synthesis reaction, also known as a direct combination reaction, occurs when two or more simple components combine to generate a more complex result.
N2 + 3H2 → 2 NH3
(b) Displacement
Ans: In a molecule, a displacement reaction occurs when an atom or a group of atoms is displaced by another atom.
Cu(s) + 2AgNO3(aq) → 2Ag(s) + Cu(NO3)2(aq)
(c) Double decomposition (precipitation)
Ans: A double decomposition process occurs when the positive and negative ions in two compounds switch partners, resulting in the formation of two new compounds.
AgNO3(aq) + NaCl(aq) →AgCl(s)+NaNO3(aq)
(d) Neutralisation of insoluble base
Ans: Insoluble base reacts with dilute acid to give soluble salt and water
CuO + H2SO4 → CuSO4 + H2O
(e) Neutralisation of an alkali (titration)
Ans: A chemical process in which an acid and a base react quantitatively with one other is known as neutralisation.
4. How would you prepare:
(a) Copper (II) sulphate from a mixture of charcoal and black copper oxide:
Ans: Because of the carbon in the charcoal, the black copper oxide is reduced to reddish-brown copper. If the lid is removed before the crucible has cooled, the hot copper will be re-oxidized by air.
In a beaker, boil dilute sulphuric acid over wire gauze. Add little amounts of cupric oxide at a time, stirring constantly until no more dissolves and the surplus compound sinks to the bottom.
Filter it while it is still hot, then collect the filtrate in a china dish. Allow the filtrate to cool and collect the copper sulphate pentahydrate crystals after evaporating it to the point of crystallisation.
CuO + H2SO4 → CuSO4 + H2O
CuSO4 + 5H2O → CuSO4.5H2O
(b) Zinc sulphate crystals from zinc dust (powdered zinc and zinc oxide)
Ans: In a beaker, boil dilute sulphuric acid over wire gauze. With continual swirling, add several granular zinc bits. Add until the Zinc has settled to the bottom of the beaker. Efflorescence is caused by the release of hydrogen gas. When effervescence ceases, it means that all of the acid has been consumed.
Zn + H2SO4 → ZnSO4 + H2
ZnSO4 + 7H2O → ZnSO4.7H2O
(c) Sodium hydrogen carbonate crystal
Ans: In a flask, dissolve 5 grams of anhydrous sodium carbonate in approximately 25 mL distilled water. Keep the flask in a freezing mixture to cool the solution. Fill the solution with carbon dioxide gas. Sodium bicarbonate crystals will precipitate out after a while. Filter the crystals and dry them in filter paper folds.
Na2CO3 + CO2 + H2O → 2NaHCO3
(d) Calcium sulphate from calcium carbonate
Ans: By decomposition of calcium carbonates by acids. Dissolve calcium carbonate in sulphuric acid, you will get CaSO4 salt.
CaCO3 + H2SO4 → CaSO4 + CO2 + H2O
5. The following is a list of methods for the preparation of salts.
A- direct combination of two elements.
B- reaction of a dilute acid with a metal.
C- reaction of a dilute acid with an insoluble base.
D- titration of a dilute acid with a solution of soluble base.
E- reaction of two solutions of salts to form a precipitate.
Choose from the above list A to E, the best method of preparing salts by giving a suitable equation in each case:
1. Anhydrous ferric chloride
Ans: A (Direct combination of two elements)
2Fe + 3Cl2 ⟶ 2FeCl3
2. Lead chloride
Ans: E (Reaction of two solutions of salts to form a precipitate
Pb (NO3)2 + 2HCl ⟶ PbCl2 + 2HNO3
3. Sodium sulphate
Ans: D (Titration of dilute acid with a solution of soluble base)
2NaOH + H2SO4 ⟶ Na2SO4 + 2H2O
4. Copper sulphate
Ans: C (reaction of dilute acid with an insoluble base)
Cu(OH)2 + H2SO4 ⟶ CuSO4 + 2H2O
6. Name:
(a) A chloride which is insoluble in cold water but dissolves in hot water.
Ans: Lead chloride
(b) A chloride which is insoluble.
Ans: Silver chloride
(c) Two sulphates which are insoluble.
Ans: Barium sulphate and lead sulphate
(d) A basic salt.
Ans: Sodium carbonate
(e) An acidic salt.
Ans: calcium chloride
(f) A mixed salt.
Ans: Sodium potassium carbonate
(g) A complex salt.
Ans: Sodium argento cyanide
(h) A double salt.
Ans: Potash alum
7. Fill in the blanks with suitable words:
An acid is a compound which when dissolved in water forms hydronium ions as the only ________ ions. A base is a compound which is soluble in water contains _______ ions. A base reacts with an acid to form a _______ and water only. This type of reaction is known as _______.
Ans: An acid is a compound which when dissolved in water forms hydronium ions as the only positively charged ions. A base is a compound which is soluble in water containing hydroxide ions. A base reacts with an acid to form a salt and water only. This type of reaction is known as neutralisation.
8. What would you observe when:
(a) Blue litmus is introduced into a solution of hydrogen chloride gas.
Ans: Blue litmus will turn into red which will indicate the solution to be acidic.
(b) Red litmus paper is introduced into a solution of ammonia in water.
Ans: Red litmus paper will turn blue.
(c) Red litmus paper is introduced in caustic soda solution.
Ans: Red litmus will turn into blue will indicate the solution to be basic.
9. Explain why:
(a) It is necessary to find out the ratio of reactants required in the preparation of sodium sulphate.
Ans: Filtration cannot remove an excess of sodium hydroxide or sulphuric acid because they are both soluble. As a result, the ratio of solutions of the two reactants must be determined on a tiny scale.
(b) Fused calcium chloride is used in the preparation of FeCl3?
Ans: The anhydrous form of ferric chloride is extremely deliquescent, which means it collects moisture from the air and dissolves in the absorbed water to produce a solution. Fused calcium chloride is used to prepare ferric chloride in order to keep it dry.
(c) Anhydrous FeCl3 cannot be prepared by heating hydrated iron (III) chloride.
Ans: When the hydrate is heated, the HCl acid is removed, leaving just the basic salt (FeOCl) or ferric oxide. As a result, heating the hydrate will not produce anhydrous ferric chloride.
10. Give the preparation of the salt shown in the left column by matching with the methods given in the right column. Write the balanced equation for each preparation.
Salt | Method of Preparation |
Zinc sulphide | precipitation |
Ferrous sulphide | oxidation |
Barium sulphate | Displacement |
Ferric sulphate | Neutralisation |
Sodium sulphate | Synthesis |
Ans: Zinc Sulphate – Displacement Zn(OH)2 + H2SO4 ⟶ ZnSO4 + 2H2O
Ferrous sulphide – synthesis Fe + S ⟶ FeS
Barium sulphate – Precipitation BaCI2+H2SO4 ⟶ BaSO4 + 2HCI
Ferric sulphate – Oxidation Fe + H2SO4 ⟶ FeSO4 + H2
Sodium sulphate – Neutralisation 2NaOH + H2SO4 ⟶ Na2SO4 + 2H2O
11. (a) Give the pH value of pure water. Does it change if common salt is added to it?
Ans: The pH value of pure water is 7. Common salt is neutral in nature. Therefore, no change in pH will occur, when common salt is added to it.
(b) Classify the following solutions as acids, bases or salts.
Ammonium hydroxide, barium chloride, sodium chloride, sodium hydroxide, H2SO4 and HNO3.
Ans:
Acid | Base | Salt |
H2SO4 | Ammonium hydroxide | Barium chloride |
HNO3 | Sodium hydroxide | Sodium chloride |
12. Define the term neutralisation:
Ans: Neutralization is the process by which H+ ions of an acid react completely with the [OH]- ions of a base to give salt and water only.
(a) Give a reaction, mentioning clearly acid and base used in the reaction.
Ans: NaOH + HCl ⟶ NaCI + H2O
(b) If one mole of a strong acid reacts with one mole of a strong base, the heat produced is always the same. Why?
Ans: Neutralization is simply a reaction between H+ ions given by strong acid and OH- ions given by a strong base. In the case of all strong acids and strong bases, the number of H+ and OH- ions produced by one mole of a strong acid or strong base is always the same. Hence the heat of neutralization of a strong acid with a strong base is always the same.
13. Write the balanced equations for the preparation of the following salts in the laboratory:
(a) A soluble sulphate by the action of an acid on an insoluble base.
Ans: MgCO3 + H2SO4 → MgSO4 + H2O + CO2
(b) An insoluble salt by the action of an acid on another salt.
Ans: Pb(NO3)2 + H2SO4 → PbSO4 + 2HNO3
(c) An insoluble base by the action of a soluble base on a soluble salt.
Ans: Pb(NO3)2 + Na2CO3 → PbCO3 + 2NaNO3
(d) A soluble sulphate by the action of an acid on a metal.
Ans: Zn + H2SO4 → ZnSO4 + H2
14. You are provided with the following chemicals:
NaOH, Na2CO3, H2O, Zn(OH)2, CO2, HCl, Fe, H2SO4, Cl2, Zn. using the suitable chemicals from the given list only, state briefly how you would prepare:
(a) Iron (III) chloride
Ans: A direct combination of elements produces iron chloride.
2Fe + 3Cl2 → 2FeCl3
(b) Sodium sulphate
Ans: By caustic soda neutralisation with dil. Sulphuric acid.
2NaOH + H2SO4 → Na2SO4 + 2 H2O
(c) Sodium zincate
Ans: By the action of alkali on metals.
Zn + 2NaOH → Na2ZnO2 + H2
(d) Iron (II) sulphate
Ans: It can be prepared by the action of dil. Acid on any Iron (active metal)
Fe + H2SO4 → FeSO4 + H2
(e) Sodium chloride
Ans: By the neutralisation reaction of HCl and NaOH.
NaOH + HCl → NaCl + H2O
15. For each of the salt: A, B, C and D, suggest a suitable method of its preparation.
(a) A is a sodium salt.
Ans: By the neutralisation reaction of HCl and NaOH.
NaOH + HCl → NaCl + H2O
(b) B is an insoluble salt.
Ans: Pb(NO3)2 + H2SO4 → PbSO4 + 2HNO3
(c) C is a soluble salt of copper.
Ans: CuCO3 + H2SO4 → CuSO4 + H2O + CO2
(d) D is a soluble salt of zinc.
Ans: Zn + H2SO4 → ZnSO4 + H2
16. Choosing only substances from the list given in the box below, write equations for the reactions which you would use in the laboratory to obtain:
(a) Sodium sulphate
(b) Copper sulphate
(c) Iron (II) sulphate
(d) Zinc carbonate
Dilute sulphuric acid Copper Copper Carbonate
Iron Sodium Carbonate
Sodium
Zinc
Ans:
(a) Sodium carbonate and dilute sulphuric acid
(b) Copper carbonate and dilute sulphuric acid
(c) Dilute sulphuric acid and Iron
(d) Dilute sulphuric acid, Zinc, and sodium carbonate
17. From the formula listed below, choose one, in each case, corresponding to the salt having the given description:- AgCl. CuCO3, CuSO4.5H2O, KNO3, NaCl, NaHSO4, Pb(NO3)2, ZnCO3, ZnSO4.7H2O.
(a) An acid salt.
Ans: NaHSO4
(b) An insoluble chloride
Ans: AgCl
(c) On treatment with concentrated sulphuric acid, this salt changes from blue to white.
Ans: CuSO4.5H2O
(d) On heating, this salt changes from green to black.
Ans: CuCO3
(e) This salt gives nitrogen dioxide when heated.
Ans: Pb(NO3)2
18. (a) Ca(H2PO4)2 is an example of a compound called ……….(acid salt/ basic salt/normal salt).
Ans: Acid salt
(b) Write the balanced equation for the reaction of:
A named acid and a named alkali.
Ans: NaOH + HCl → NaCl + H2O
19. State the terms defined by the following sentences:
(a) A soluble base.
Ans: Alkali
(b) The insoluble solid formed when two solutions are mixed together.
Ans: Precipitate
(c) An acidic solution in which there is only partial ionization of the state molecules.
Ans: Weak acid
20. Which of the following methods, A, B, C, D, or E is generally used for preparing the chlorides listed below from (i) to (v). Answer by writing down the chloride and the letter pertaining to the corresponding method. Each letter is to be used only once.
A. Action of an acid on a metal.
B. Action of an acid on an oxide or carbonate.
C. Direct combination.
D. Neutralization of an alkali by an acid.
E. Precipitation (double decomposition)
(i) Copper (II) chloride
Ans: Action of an acid on an oxide or carbonate.
(ii) Iron (II) chloride
Ans: Action of an acid on a metal.
(iii) Iron (III) chloride
Ans: Direct combination
(iv) Lead (II) chloride
Ans: Precipitation (double decomposition)
(v) Sodium chloride
Ans: Neutralization of an alkali by an acid.
21. Choose the most appropriate answer from [SO2, SiO2, Al2O3, CO, MgO, Na2O]
(a) A covalent oxide of a metalloid.
Ans: SiO2
(b) An oxide which when dissolved in water form acid.
Ans: SO2
(c) A basic oxide.
Ans: MgO
(d) An amphoteric oxide.
Ans: Al2O3
22. Complete the following table and write one equation for each to justify the statement:
Reactant | Products | Method |
Soluble base + Acid (dil) | Salt + water | Neutralisation Titration |
Metal + Non-metal | Salt (soluble/insoluble) | _______ |
Insoluble base + _______ | Salt (soluble) + water | _______ |
Active metal + Acid (dil) | _______ + _______ | _______ |
Soluble salt solution (A) + soluble salt solution (B) | Precipitated salt + soluble salt | _______ |
carbonate/ bicarbonate + Acid (dil) | Salt + _______ + _______ | Decomposition of carbonate |
chlorides/nitrates + Acid (dil) | _______ + _______ | Decomposition of chlorides and nitrates |
Ans:
Reactant | Products | Method |
Soluble base + Acid (dil) | Salt + water | Neutralisation Titration |
Metal + Non-metal | Salt (soluble/insoluble) | Direct combination |
Insoluble base + Acid | Salt (soluble) + water | Neutralisation |
Active metal + Acid (dil) | Salt + Hydrogen | Displacement |
Soluble salt solution (A) + soluble salt solution (B) | Precipitated salt + soluble salt | Precipitation |
carbonate/ bicarbonate + Acid (dil) | Salt+ water + carbon dioxide | Decomposition of carbonate |
chlorides/nitrates + Acid (dil) | Acid salt + HCl/HNO3 | Decomposition of chlorides and nitrates |
Exercise – 3C
1. What do you understand about water crystallisation? Give four substances which contain water of crystallisation and write their common names.
Ans: Some salts, while crystallizing out of their solutions, unite with a definite quality of water which is known as water of crystallization.
Four substances which contain water of crystallization:
Na2CO3.10H2O (Washing soda), MgSO4.7H2O (Epsom salt), K2SO4.Al2(SO4)3.24H2O (Potash alum), and Na2SO4.10H2O (Glauber's salt).
2. (a) Define efflorescence. Give examples.
Ans: Efflorescence is the property of some substances to lose wholly, or partly their water of crystallization when their crystals are exposed to dry air even for a short time.
Examples are: Washing soda, Glauber's salt, Epsom salt.
(b) Define deliquescence. Give examples.
Ans: Deliquescent compounds absorb enough water from the air to dissolve in the water they have taken up. Deliquescent substances include calcium chloride (CaCl2) and sodium hydroxide (NaOH).
3. Answer the questions below, relating your answers only to salts in the following list: Sodium chloride, anhydrous calcium chloride, copper sulphate-5-water?
(a) What name is given to the water in the compound copper sulphate-5-water?
Ans: Water of crystallisation.
(b) If copper sulphate-5-water is heated, anhydrous copper sulphate is formed. What is its colour?
Ans: White
(c) By what means, other than heating, could you dehydrate copper sulphate-5-water and obtain anhydrous copper sulphate?
Ans: By adding sulphuric acid (dehydrating agent)
(d) Which one of the salts in the given list is deliquescent?
Ans: Calcium chloride.
4. State your observations when the following are exposed to the atmosphere:
(a) Washing soda Crystals
Ans: It loses its nine water molecules (water of hydration).
(b) Iron (III) chloride salt
Ans: It will absorb moisture from the atmosphere.
5. Give reason for the following:
(a) Sodium hydrogen sulphate is not an acid but it dissolves in water to give hydrogen ions, according to the equation.
NaHSO4 ⇆ H+ + Na+ + SO4-2
Ans: Sodium hydrogen sulphate is not an acid but undergoes partial replacement of the ionisable hydrogen atom and behaves as an acidic salt to give H+ ions.
(b) Anhydrous calcium chloride is used in a desiccator.
Ans: As calcium chloride absorbs moisture and keeps the compound dry, it is used in desiccators as a drying agent.
6. Explain clearly how conc. H2SO4 is used as a dehydrating as well as drying agent.
Ans: Because conc. sulphuric acid is hygroscopic and can absorb moisture from other materials, it is employed as a drying agent. It's also utilised as a dehydrating agent because of its high affinity for water, which allows it to swiftly absorb water from compounds.
7. Distinguish between drying and dehydrating agents.
Ans: Dehydrating agents are used to remove the water of crystallisation from chemically bound compounds, while drying agents are used to remove the excess water in the reaction mixture to produce a dry product.
8. State whether a sample of each of the following would increase or decrease in mass if exposed to air.
(a) Solid NaOH
Ans: Increases
(b) Solid CaCl2
Ans: Increase
(c) Solid Na2CO3.10H2O
Ans: Decrease
(d) Conc. Sulphuric acid
Ans: Increases
(e) Iron (III) Chloride
Ans: Increases
9. (a) Why does common salt get wet during the rainy season?
Ans: Common salt contains impurities like magnesium chloride, which are deliquescent substances. So on exposure to air especially during the rainy season, table salt turns moist though sodium chloride is not deliquescent.
(b) How can this impurity be removed?
Ans: This impurity can be removed by passing a current of dry hydrogen chloride gas through a saturated solution of the affected salt. Pure sodium chloride is produced as a precipitate, which can be recovered by filtering and washing first with water and then with alcohol.
(c) Name a substance which changes the blue colour of copper sulphate crystals to white.
Ans: Conc. H2SO4 can change the blue colour of copper sulphate to white.
(d) Name two crystalline substances which do not contain water of crystallisation.
Ans: Two crystalline substances which do not contain water of crystallization are: Common salt, Nitre, Sugar.
10. Name the salt which on hydrolysis forms
(a) Acidic solution
Ans: Ammonium chloride; NH4Cl + H2O NH4OH + HCl
(b) Basic solution
Ans: Sodium carbonate; Na2CO3 + H2O NaOH + H2CO3.
(c) Neutral solution. Give a balanced chemical equation for each reaction.
Ans: Sodium chloride; NaCl + H2O NaOH + HCl.
11. State the change noticed when blue litmus and red litmus are introduced in the following:
(a) Na2CO3 solution
Ans: No change in colour.
(b) NaCl solution
Ans: No change in colour.
(c) NH4NO3
Ans: Blue litmus will change its colour to red.
(d) MgCl2 Solution
Ans: No change in colour.
Miscellaneous Questions Based on Icse Examinations
1. Write the balanced equations for the preparation of the following compounds (as the major product) starting from iron using only one other substances:
(a) Iron (II) chloride
Ans: Fe + HCl (dil) → FeCl2 + H2
(b) Iron (III) chloride
Ans: 2Fe + 3Cl2 (dry) → FeSO4 + H2 ______(on heating)
(c) Iron (II) sulphate
Ans: Fe + H2SO4 → FeSO4 + H2
(d) Iron (II) sulphide
Ans: Fe + S → FeS (on heating)
2. Write balanced reactions for the following conversions (A, B, C, D)
\[Fe\xrightarrow{A}FeC{{l}_{2}}\xrightarrow{B}FeC{{O}_{3}}\xrightarrow{C}Fe{{(N{{O}_{3}})}_{2}}\xrightarrow{D}Fe{{(OH)}_{2}}\]
Ans:
A= HCl
B= Na2CO3
C= HNO3
D= NaOH
3. The preparation of lead sulphate from lead carbonate is a two-step process. (Lead sulphate cannot be prepared by adding dilute sulphuric acid to lead carbonate.)
(a) What is the first step that is required to prepare lead sulphate from lead carbonate?
Ans: The first step is to convert insoluble lead carbonate into soluble lead nitrate by treating lead carbonate with dilute nitric acid.
(b) Write the equation for the reaction that will take place when this first step is carried out.
Ans: PbCO3(s) + 2HNO3(dil) ⟶ Pb(NO3)2 (aq) + H2O (l) + CO2 ↑
(c) Why is the direct addition of dilute sulphuric acid to lead carbonate an impractical method of preparing lead sulphate?
Ans: When dilute sulphuric acid is added directly to lead carbonate, the lead sulphate thus formed will be deposited on solid lead carbonate disconnecting lead carbonate from sulphuric acid.
4. (a) What are the term defined by the following?
(i) A salt containing a metal ion surrounded by other ions or molecules.
Ans: Complex salt
(ii) A base which is soluble in water.
Ans: Alkali
(b) Making use only of substances chosen from those given below:
Dilute sulphuric acid Sodium carbonate
Zinc Sodium sulphite
Lead Calcium carbonate
Give the equations for the reactions by which you could obtain:
(i) Hydrogen
Ans: Zn +H2SO4 → ZnSO4 + H2
(ii) sulphur dioxide
Ans: Na2SO3 → Na2O + SO2 (on heating)
(iii) carbon dioxide
Ans: Na2CO3 +H2SO4 → Na2SO4 + H2O + CO2 (on heating)
(iv) zinc carbonate (two step required)
Ans: Zn + H2SO4 → ZnSO4 + H2
ZnSO4 + Na2CO3 → Na2SO4 + ZnCO3
2009
(a) The acid which contains four hydrogen atoms ________
(i) Formic acid
(ii) Sulphuric acid
(iii) Nitric acid
(iv) Acetic-acid
Ans: Acetic acid
(b) A black coloured solid which on reaction with dilute sulphuric acid forms a blue coloured solution is
(i) Carbon
(ii) Manganeses [IV] oxide
(iii) Lead [II] oxide
(iv) Copper [II] oxide
Ans: Copper [II] oxide
(c) Solution A is strong acid B weak acid C strong alkali
(i) Which solution contains solute molecules in addition to water molecules?
Ans: Solution B
(ii) Which solution gives gelatinous white ppt with zinc sulphate, ppt disappears in excess
Ans: Solution C
(iii) Give an example of a weak alkali.
Ans: Ammonium hydroxide
(d) Write the equations for the reaction to prepare lead sulphate from lead carbonate.
Ans: H2SO4 + PbCO3 → PbSO4 + H2O + CO2.
(e) Define the following term- neutralization.
Ans: Neutralization is a reaction, in which acid and base react with each other and form salt and water.
(f) The diagram given below is to prepare Iron [III] chloride in the laboratory:
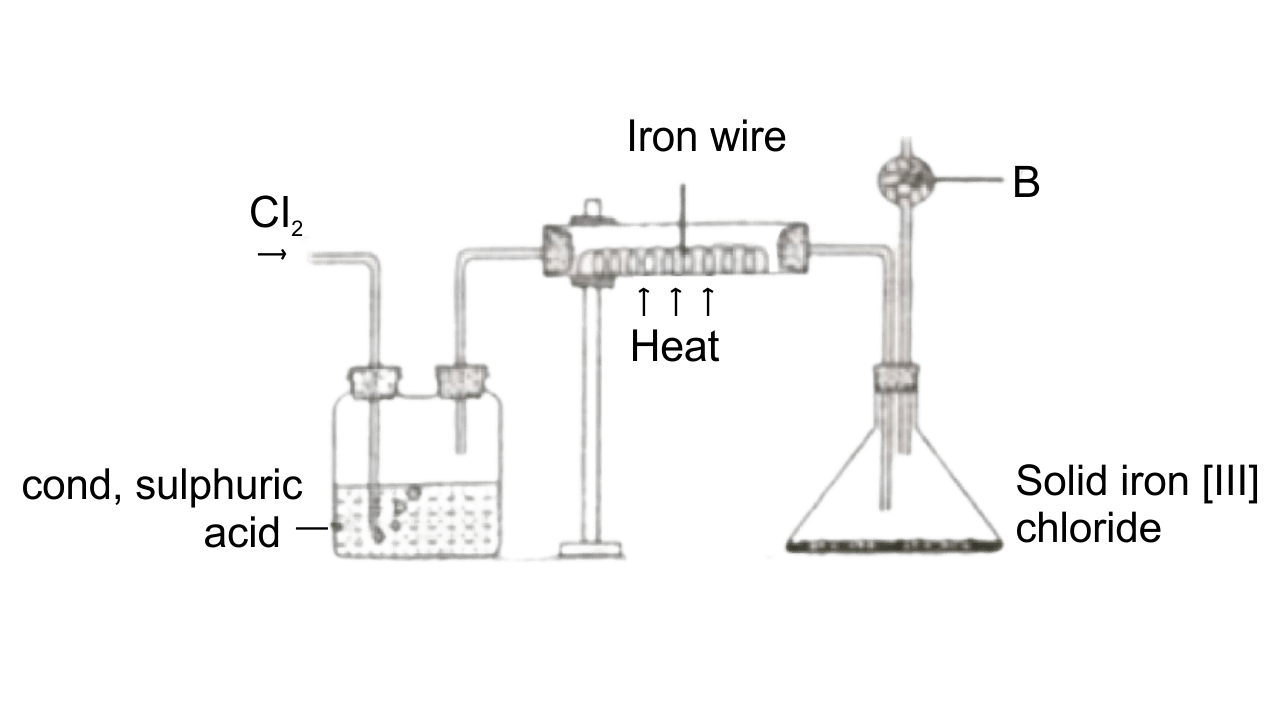
(i) What is substance B.
Ans: Anhydrous calcium chloride
(ii) What is the purpose of B.
Ans: Acting as drying agent.
(iii) Why is iron [III] chloride stored in a closed container?
Ans: iron [III] chloride is a deliquescent substance. Therefore, they need to be stored in a closed container.
(iv) Write the equation for the reaction between iron and chlorine.
Ans: 2Fe + 3Cl2 → 2FeCl3
2010
(a) Select the correct answer from A, B, C, D and E-
A. Nitroso iron [II] sulphate
B. Iron [III] chloride
C. Chromium sulphate
D. Lead chloride
E. Sodium chloride
(i) A deliquescent compound
Ans: B. Iron [III] chloride
(ii) A compound soluble in hot water but insoluble in cold water.
Ans: Lead chloride
(iii) A compound which in the aqueous solution state, is neutral in nature.
Ans: Sodium chloride
(b) Select the correct answer from A, B, C and D-
(i) A weak organic acid is:
A. Formic acid
B. Sulphuric acid
C. Nitric acid
D. Hydrochloric acid
Ans: Formic acid
(ii) A complex salt is
A. Zinc sulphate
B. Sodium hydrogen sulphate
C. Iron [ammonium sulphate]
D. Tetrammine copper [II] sulphate
Ans: Tetrammine copper [II] sulphate
(c) Give equations for the following conversions A to E-
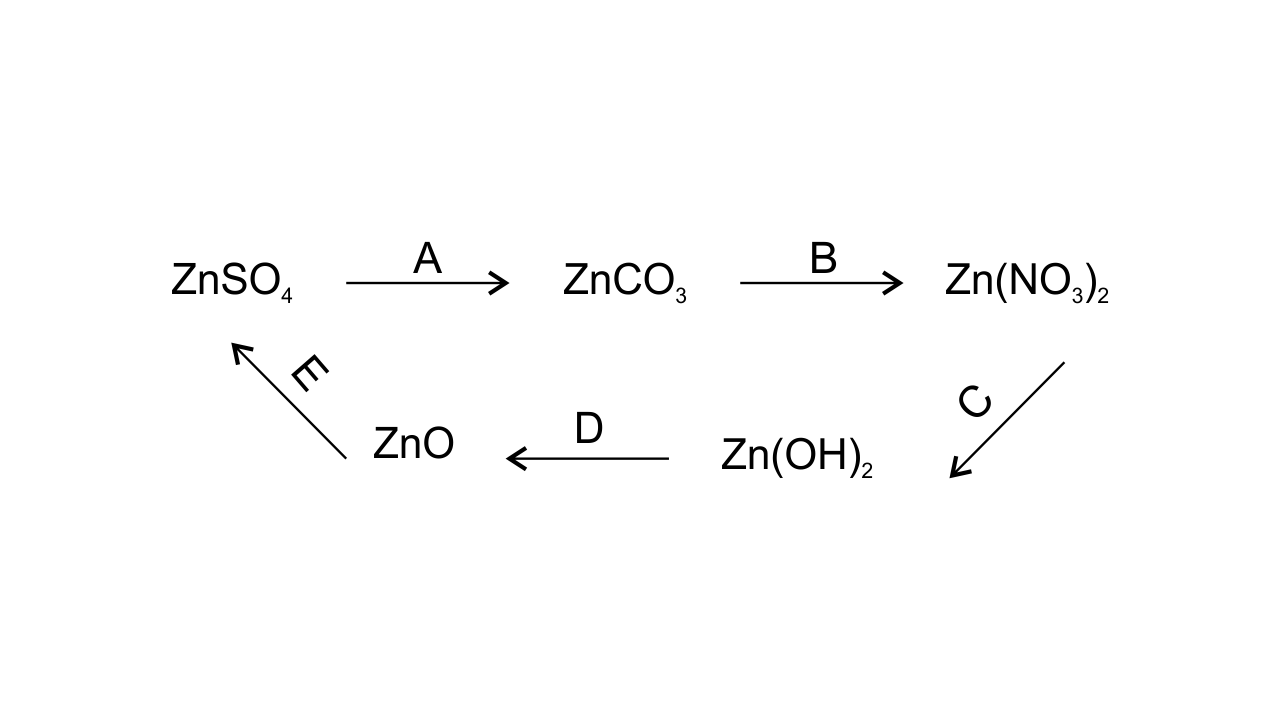
Ans:
A. ZnSO4 + Na2CO3 → ZnCO3 + Na2SO4
B. ZnCO3 + 2HNO3 → Zn (NO3)2
C. Zn (NO3)2 + 2NaOH → Zn (OH)2
D. Zn (OH)2 → ZnO + H2O
E. ZnO + H2SO4 → ZnSO4
(d) For the preparation of the following salts- give a balanced equation in each case.
(i) Copper [II] sulphate from copper [II] oxide
Ans: CuO + H2SO4 → CuSO4 + H2O
(ii) Iron [III] chloride from the metal iron
Ans: 2Fe + 3Cl2 → 2FeCl3
(iii) Potassium sulphate from KOH solution
Ans: KOH + H2SO4 → K2SO4 + H2O
(iv) Lead [II] chloride from lead carbonate
Ans: PbCO3 + HCl → PbCl2 + H2O + CO2
2011
(a) Write the balanced chemical equation- Lead nitrate solution is added to sodium chloride solution.
Ans: PbNO3 + NaCl → PbCl2 + NaNO3
(b) State what happens to the crystal of washing soda when exposed to air. Name the phenomenon exhibited.
Ans: When washing soda crystals are exposed to air, they lose their water of crystallisation and become amorphous. Efflorescence is the term for this phenomenon.
(c) Name the method used for the preparation of the following salts from the list given below:
(i) sodium nitrate
(ii) Iron (III) chloride
(iii) Lead chloride
(iv) Zinc sulphate
(v) Sodium hydrogen sulphate
List:
(A) Simple displacement
(B) Neutralisation
(C) Decomposition by acid
(D) Double decomposition
(E) Direct synthesis
Ans:
(i) Sodium nitrate - (B) Neutralisation.
(ii) Iron (III) chloride - (E) Direct Synthesis.
(iii) Lead chloride - (D) Double decomposition
(iv) Zinc sulphate - (A) Simple displacement.
(v) Sodium hydrogen sulphate - (C) Decomposition by acid.
2012
(a) Match the following:
Column A | Column B |
(i) Acid salt | (A) Ferrous ammonium sulphate |
(ii) Double salt | (B) Contains only ions |
(iii) Ammonium hydroxide solution | (C) Sodium hydrogen sulphate |
(iv) Dilute hydrochloric acid | (D) Contains ions and molecules |
(v) Carbon tetrachloride | (E) contains only molecules |
Ans:
Column A | Column B |
(i) Acid salt | Sodium hydrogen sulphate |
(ii) Double salt | Ferrous ammonium sulphate |
(iii) Ammonium hydroxide solution | Contains ions and molecules |
(iv) Dilute hydrochloric acid | Contains only ions |
(v) Carbon tetrachloride | contains only molecules |
(b) State your observation: Zinc granules are added to copper sulphate solution.
Ans: zinc displaces copper to form zinc sulphate and thus copper gets deposited.
(c) Give a balanced equation for the reaction: Silver nitrate solution and sodium chloride solution.
Ans: AgNO3 + NaCl → NaNO3 + AgCl
2013
(a) Select the words given which are required to correctly complete the blanks-
[Ammonia, ammonium carbonate, carbon dioxide, hydrogen, hydronium, hydroxide, precipitate, salt, water]
(i) A solution M turns blue litmus red, so it just contains (1) _______ Ions; another solution turns red litmus blue and hence, must contain (2) _______ ions.
Ans:
(1) Hydronium
(2) Hydroxide
(ii) When solution M and O are mixed together, the products will be (3) ______ and (4) ______
Ans:
(3) Salt
(4) water
(iii) If a piece of magnesium was put into a solution M. (5) _______ gas would be evolved.
Ans: (5) Hydrogen
(b) Give a suitable chemical term for:
(i) A salt formed by incomplete neutralisation of an acid by a base.
Ans: Acidic salt
(ii) A definite number of water molecules bound to some salts.
Ans: water of crystallisation
(iii) The process in which a substance absorbs moisture from the atmospheric air to become moist, and ultimately dissolves in the absorbed water.
Ans: Deliquescence
(c) Choosing the substances from the list given: dil. Sulphuric acid, copper, iron, sodium copper [II] carbonate, sodium carbonate, sodium chloride, zinc nitrate.
Write balanced equations for the reactions which would be used in the laboratory to obtain the following salts:
(i) Sodium sulphate
Ans: Na2CO3 + H2SO4 → Na2SO4 + H2O + CO2
(ii) Zinc carbonate
Ans: Zn(NO3)2 + Na2CO3 → ZnCO3 + 2NaNO3
(iii) Copper [II] sulphate
Ans: CuCO3 + H2SO4 → CuSO4 + H2O + CO2
(iv) Iron [II] sulpahte
Ans: Fe + H2SO4 → FeSO4 + H2
(d) Which one of the following will not produce acid with water
(i) CO
(ii) CO2
(iii) NO2
(iv) SO3
Ans: CO
2014
(a) Fill in the blank from the choices given: The basicity of the acetic acid is _____ [3, 1, 4].
Ans: Acetic acid has only one replaceable hydrogen ion per molecule, or you might say it only makes one hydrogen ion per molecule. As a result, acetic acid has a basicity of 1 or is a monobasic acid.
(b) Draw the structure of the stable positive ion formed when an acid dissolves in water.
Ans:
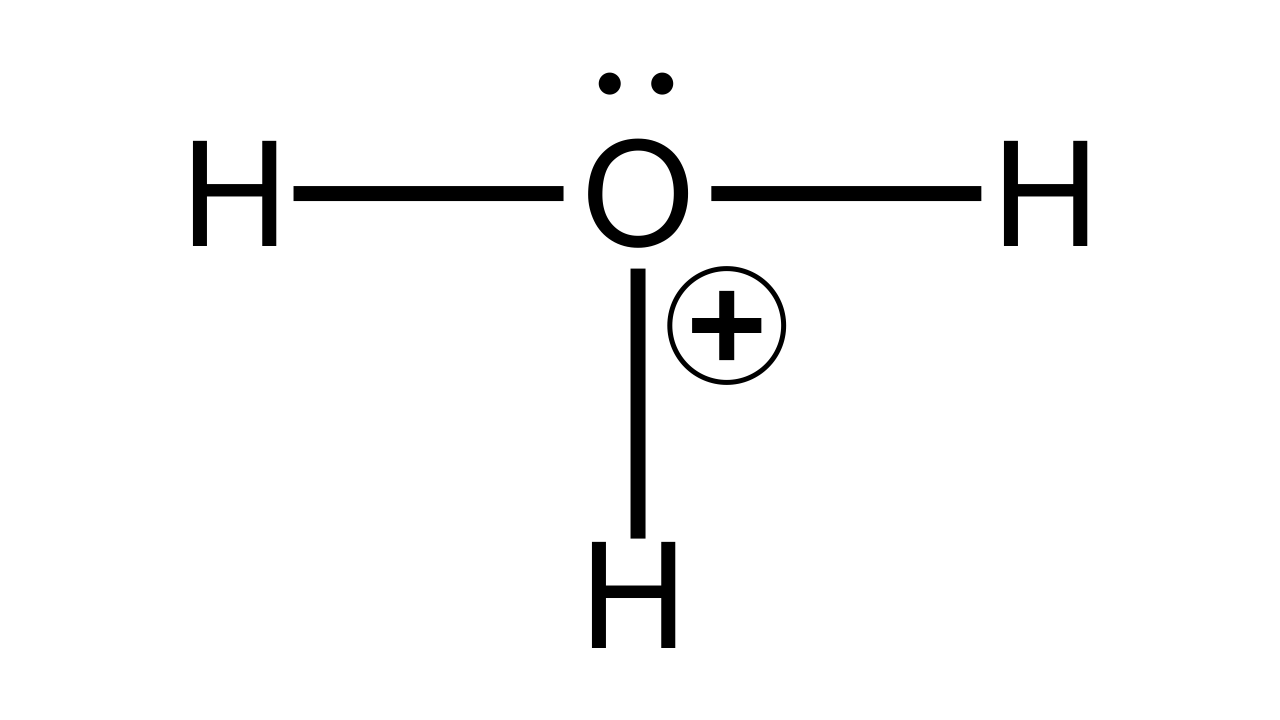
(c) State the inference drawn from the observation:
Ans: Salt S is prepared by reacting dilute sulphuric acid with copper oxide. Identify S. Ans: Salt S is made by combining copper oxide and dilute sulphuric acid. As a result, salt S is copper sulphate.
(d) Give balanced chemical equations for the preparation of the following salts:
(i) Lead sulphate- from lead carbonate
Ans: PbCO3 + 2HNO3 → Pb(NO3)2 + H2O + CO2
Pb(NO3)2 + H2SO4 → PbSO4 + 2HNO3
(ii) Sodium sulphate- using dilute sulphuric acid
Ans: 2NaOH + H2SO4 → Na2SO4 + 2H2O
(iii) Copper chloride- using copper carbonate
Ans: CuCO3 + 2HCl → CuCl2 + H2O + CO2
2015
(a) From the list of salts- Agcl, MgCl2, NaHSO4, PbCO3, ZnCO3, KNO3, Ca(NO3)2
Choose the salt that most appropriately fits the description given below:
(i) A deliquescent salt
Ans: MgCl2
(ii) An insoluble chloride
Ans: AgCl
(b) From- Na2O, SO2, SiO2, Al2O3, MgO, CO, select an oxide which dissolves in water forming an acid.
Ans: SO2
2016:
Match the salts given in column I with their method of preparation given in column II.
Column I | Column II |
(i) Pb(NO3)2 from PbO | (a) Simple displacement |
(ii) Mgcl2 from Mg | (b) Titration |
(iii) FeCl3 from Fe | (c) Neutralization |
(iv) NaNO3 from NaOH | (d) Precipitation |
(v) ZnCO3 from ZnSO4 | (e) Combination |
Ans:
Column I | Column II |
Pb(NO3)2 from PbO | Precipitation |
Mgcl2 from Mg | Simple displacement |
FeCl3 from Fe | Combination |
NaNO3 from NaOH | neutralization |
ZnCO3 from ZnSO4 | Titration |
Free download of step by step solutions for class 10 Science (Chemistry) chapter 3 - Acids, Bases and Salts of ICSE Board (Concise - Selina Publishers). All exercise questions are solved & explained by an expert teacher and as per ICSE board guidelines.
Advantages of Studying with Vedantu
Here are the advantages of studying with Vedantu:
The study materials provided by Vedantu are completely reliable and easy to understand.
Step by step solutions to every problems are provided.
The study materials are prepared by the experts of Vedantu after a thorough research.
The study materials are available online 24 by 7 and also, at free of cost.
All the materials are according to the latest ICSE syllabus and guidelines.
FAQs on Acids, Bases and Salts Solutions for ICSE Board Class 10 Science
1. What is Bronsted-Lowry Theory?
The Bronsted-Lowry theory, which is also known as theProton theory of acid and base is an acid-base reaction theory. In the year 1923, this theory was introduced to us by a Danish Chemist, who was Johannes Nicolaus Bronsted and an English Chemist, who was Thomas Martin Lowry.
According to this theory, acid and base react with each other because of the interchanging of the proton acid, which forms its conjugate base, which then forms conjugate acid.
The Bronsted-Lowry theory is an extended form of an Arrhenius theory of acid-base.
In the Arrhenius theory, it is seen that, in a liquid solution, acid elevates the concentration of H+ ions while the base elevates the concentration of OH– ions. The challenge of Arrhenius theory was, it recognizes the reaction of an acid and base only in the liquid medium.
2. What is an acid and base in Chemistry?
Solutions are classified as acidic or introductory grounded on their hydrogen ion attention relations to pure water. Acidic results have an advanced H+ attention than water, while introductory (alkaline) results have a lower H attention. Generally, the hydrogen ion attention of a result is expressed in terms of PH.
H+ attention shifts down from neutral when a base or acid is added to a waterless (water-grounded) result. An acid can be described as a substance that either gives away protons or accepts valence electrons to form a bond. While the base is a substance that behaves in an opposite manner when compared to acid. It accepts the proton and gives away valence electrons.
The stronger the acid, the more readily it dissociates to induce H+. For illustration, hydrochloric acid (HCI) fully dissociates into hydrogen and chloride ions when it's placed in water, so it's considered a strong acid. The acids in tomato juice or ginger, on the other hand, don't fully disconnect water and are considered weak acids. Also, strong bases like sodium hydroxide (NaOH) fully disconnect in water, releasing hydroxide ions (or other types of introductory ions) that can absorb H.
3. What is a Buffer?
Most of the organisms, including humans, need to maintain pH within a fairly narrow range in order to survive. For example, mortal blood requires to keep its pH right around7.4 and avoid shifting significantly advanced or lower – indeed if acidic or introductory substances enter or leave the bloodstream.
Buffers, results that can repel changes in pH, are crucial to maintaining stable H ion attention in natural systems. When there are too numerous H ions, a buffer will absorb some of them, bringing pH back over; and when there are too many, a buffer will contribute some of its own H ions to reduce that.
Buffers generally correspond to an acid-base, with the acid and base differing by the presence or absence of a portion (a conjugate-acid base brace).
For example, one of the buffers that maintain the pH of mortal blood involves carbonic acid and its conjugate base, the bicarbonate ion.
Carbonic acid is created when carbon dioxide enters the bloodstream and mixes with water, and it's the main form in which carbon dioxide peregrination in the blood between the muscles (where it's generated) and the lungs (where it's converted back into water and carbon dioxide), which is released as a waste product.
4. What is a pH scale?
The pH scale is used to rank results in terms of acidity or stipulation (alkalinity). Since the scale is grounded on pH values, it's logarithmic, meaning that a change of 1 pH unit corresponds to an aten-fold change in H ion attention. The pH scale is frequently said to range from 0 to 14, and utmost results do fall within the range, although it's possible to get a pH below 0 or over 14. Anything below7.0 is acidic, and anything above7.0 is alkaline, or introductory.
The pH inside mortal cells (6.8) and the pH of blood (7.4) are both close to neutral. Extreme pH values, either above or below7.0, are generally considered inimical for life. Still, the terrain inside your stomach is largely acidic, with a pH of 1 to 2.
5. What is Hydrogen Bonding?
Hydrogen bonding is the conformation of Hydrogen bonds. This occurs due to the intermolecular forces that are attracted and take place because of the dipole-dipole activity between hydrogen atoms.
Hydrogen bonds are substantially strong in comparison to normal dipole-dipole and dissipation forces. Still, they're weak compared to true covalent or ionic bonds.



































