Overview of Periodic Table, Periodic Properties and Variations of Properties Solutions for ICSE Board Class 10 Chemistry
Free download of step-by-step solutions for class 10 Chemistry chapter 1 - Periodic Table, Periodic Properties and Variations of Properties of ICSE Board (Concise - Selina Publishers). All exercise questions are solved & explained by an expert teacher and as per ICSE board guidelines.
Selina’s textbook students learn about the periodic table in detail in chapter 1 of class 10 Chemistry. They also learn about the relationship between the atomic properties of the elements and their position on the periodic table. Because the periodic table is one of the most important topics in Chemistry, we have provided free Concise Chemistry Class 10 ICSE Solutions for Chapter 1 to help students fully understand all of the concepts, including the definitions and trends of periodic properties.
Furthermore, the solutions provided here include detailed answers to all of the difficult questions presented in the chapter. Our experts carefully prepared the solutions for the Selina Concise Chemistry textbook, which will assist students in studying in an easy and objective manner. In essence, using these solutions will allow students to effectively prepare for and achieve better results in the upcoming ICSE board exams.
Access ICSE Selina Solutions for Grade 10 Chemistry Chapter 1 – Periodic Table, Periodic Properties and variations of Properties
1. (i) State modern periodic law.
Ans: "The properties of elements are periodic functions of their atomic number," according to the modern periodic law.
(ii) Name the scientist who stated the law.
Ans: The periodic law was proposed by Henry Moseley.
(iii) How many groups and periods does modern periodic table have?
Ans: Modern Periodic table has 7 periods and 18 groups.
2. What are horizontal rows and vertical columns in a periodic table known as?
Ans: In the periodic table, the horizontal rows are known as periods, while the vertical columns are known as groups.
3. Periodicity is observed due to the similar.............
(number of valence electrons/ atomic number/ electronic configuration)
Ans: Periodicity is observed due to the similar electronic configuration.
4. How does electronic configuration in atoms change
(i) In a period from left to right?
Ans: While the number of shells remains constant, the number of valence electrons rises by one as we move from left to right over any given period.
(ii) In a group top to bottom?
Ans: The number of shells rises sequentially, one by one, from top to bottom in a group, but the number of valence electrons remains constant.
5. Name 2 elements in each case:
(i) Alkali metals
Ans: Sodium and Potassium
(ii) Alkaline earth metals
Ans: Calcium and Magnesium
(iii) Halogens
Ans: Chlorine and Bromine
(iv) Inert gas
Ans: Neon and Argon
6. Elements of group 1 and elements of group 17 both have valency1. Explain.
Ans: The combining capability of an element's atom is known as valency. It's the number of electrons that an atom can provide, take, or share. It's merely a number with no positive or negative sign attached to it.
The outermost orbital of Group 1 elements has one electron, while the outermost orbital of Group 7 elements has seven electrons. The number of electrons in the outermost shell determines the valency (i.e. valence shell). If the outermost shell has only one electron, it can contribute one electron while interacting with other elements to form a stable electronic configuration. If the outermost shell has seven electrons, its valency is one (8 - 7 = 1) since it may accept one electron from the combining atom. The number of electrons in the valence (outermost) shell rises from left to right in a given period. However, the valency grows only until Group 14, when it becomes 4, and then drops, becoming 1 in Group 17.
7. Correct the statements.
(i) Elements in the same period have equal valency.
Ans: Elements in the same group have equal valency.
(ii) Valency depends upon the number of shells in an atom.
Ans: Valency depends upon the number of valence electrons in an atom.
(iii) Copper and zinc are representative elements.
Ans: Copper and zinc are transition elements.
(iv) Transition elements are placed at the extreme right of the periodic table.
Ans: Noble gases are placed at the extreme right of the periodic table.
8. What do you understand by?
(i) Periodicity
Ans: Periodic qualities are properties that return at regular intervals or have a progressive variation at regular intervals, and the phenomenon is known as periodicity of elements.
(ii) Typical elements
Ans: The third-period elements Na, Mg, Al, Si, P, and Cl are known as typical elements because they summarise the features of their respective groups.
(iii) Orbits
Ans: The orbits or shells in which elements circle around the nucleus are known as orbits or shells.
9. Name 2 elements you would expect to show chemical reactions similar to calcium. What is the basis of your choice?
Ans: Beryllium and magnesium will have chemical interactions that are similar to those of calcium. Because these elements, like calcium, belong to the same group 2 and have two electrons in their outermost shell.
10. Name the (i) metals, (ii) metalloids and (iii) non-metals in the first twenty elements.
Ans: Metals: Lithium, Beryllium, Sodium, Magnesium, Aluminium, Potassium, Calcium
Metalloids: Boron, Silicon
Non-metals: Hydrogen, Helium, Carbon, Nitrogen, Oxygen, Fluorine, Neon, Phosphorous, Sulphur, Chlorine, Argon
11. Fluorine, Chlorine and Bromine are put in one group on the basis of their similar properties.
(i) What are those similar properties?
Ans: Non-metallic, highest electronegativity, highest ionisation potentials, and maximum electron affinity in respective periods.
(ii) What is the common name of this group or family?
Ans: The common name is halogens, as they are salt forming elements.
12. What is the main characteristic of the last element in each period of the Periodic Table? What is the general name of such elements?
Ans: The last element in each period of the periodic table has the property of being inert or chemically unreactive. These elements are referred to as "Noble gases" in general.
13. According to atomic structure, what determines which element will be the first and which will be the last in a period?
Ans: The quantity of valence electrons defines the first and final element in a period, according to atomic structure.
14. How does the number of:
i. Valence electrons and
ii. Valency vary on moving from left to right in the third period of the periodic table?
Ans:
i. In the third period of the periodic table, the valence electrons grow from 1 to 8.
ii. The valency climbs from 1 to 4 and then lowers from 4 to 0 as you move from left to right.
15. Name the type of elements, which have their
(i) Outermost shell complete
Ans: Noble gases
(ii) Outermost shell incomplete
Ans: Representative elements
(iii) Two outermost shells incomplete
Ans: Transition elements
(iv) One electron short of octet
Ans: Halogens
(v) Two electrons in the outermost orbit.
Ans: Alkaline Earth metals
16. An element has 2 electrons in its N shell
(i) What is its atomic number?
Ans: 30
(ii) State its position in periodic table
Ans: It belongs to group 12 and fourth period.
(iii) Is it metal or non-metal?
Ans: It is a metal.
(iv) State the name assigned to this group.
Ans: The name assigned to this group is IIB.
(v) What is the valency of this element?
Ans: 2
17. Answer the following in respect of element 16S32.
(i) Give its electronic configuration.
Ans: Electronic configuration of Sulphur (S): 2,8,6
(ii) To which group and period does it belong?
Ans: 16th Group and 3rd Period.
(iii) What is its valency?
Ans: Valency of S = 8 - 6 = 2
(iv) Is it metal or non-metal?
Ans: Sulphur is a non-metal.
(v) Is it a reducing agent or oxidizing agent?
Ans: It is an oxidizing agent.
(vi) Give its formula with hydrogen.
Ans: Formula with hydrogen = H2S
18. Name an
A. An alkali metal in period 3 and halogen in period 2
Ans: Na and F
B. The noble gas with 3 shells
Ans: Argon
C. The non-metals present in period 2 and metals in period 3.
Ans: C, N, O and F are non-metals present in period 2 while Na, Mg and Al are metals in period 3.
D. The element of period 3 with valency 4.
Ans: Silicon
E. The element in period 3 which does not form an oxide.
Ans: Argon
F. The element of lower nuclear charge out of Be and Mg.
Ans: Mg
19. The electronic configuration of an element T is 2, 8, 8, 1.
(i) What is the group number of T?
Ans: Group = 1
(ii) What is the period number of T?
Ans: Period = 4
(iii) How many valence electrons are there in an atom of T?
Ans: Valence electrons = 1
(iv) What is the valency of T?
Ans: Valency = 1
(v) Is it a metal or a non-metal?
Ans: Metal
20. Match the atomic number 19, 15, 8, 4 and 2 with each of the following:
(i) A metal of valency one
Ans: A metal of valency one = 19
(ii) A solid non-metal of period 3
Ans: A solid non-metal of period 3 = 15
(iii) A rare gas
Ans: A rare gas = 2
(iv) A gaseous element with valency 2
Ans: A gaseous element with valency 2 = 8
(v) An element of group 2
Ans: An element of group 2 = 4
Intext Questions
1. What do you understand by atomic size? State its unit.
Ans: The distance between an atom's nucleus and its outermost shell is measured in atomic size. Angstrom and picometer are the units of measurement.
2. Give the trends in atomic size on moving
(i) Down the group
Ans: When we go along a group from top to bottom, the atomic size of an atom grows.
(ii) Across the period left to right.
Ans: As we move from left to right over time, it lowers.
3. Arrange the elements of second and third period in increasing order of their atomic size. (excluding noble gases).
Ans: Second Period: Fluorine < Oxygen < Nitrogen < Carbon < Boron < Beryllium < Lithium.
Third Period: Chlorine < Sulphur < Phosphorus < Silicon < Aluminum < Magnesium < Sodium.
4. Why is the size of
(i) Neon greater than fluorine
Ans: Because neon has a complete outer shell, its size is larger than that of fluorine (octet). As a result, the effect of nuclear attraction on electrons in the valence shell is invisible. As a result, Neon is larger than fluorine.
(ii) Sodium is greater than magnesium.
Ans: Because magnesium has a higher atomic number than sodium but the same number of shells, Mg atoms have a stronger nuclear pull. As a result, it is smaller than sodium.
5. (i) Which is greater in size
(a) An atom or a cation?
(b) An atom or an anion?
(c) Fe2+ or Fe3+?
Ans: Because a cation is generated by the loss of electrons, an atom is always larger than a cation; thus, protons in a cation are greater than electrons. As a result, the nucleus attracts the electrons and pulls them inward.
An anion is larger than an atom because it is generated by the gain of electrons, which means there are more electrons than protons. Because the nucleus' effective positive charge is lower, there is less inward attraction. As a result, the size expands. Fe2+ is larger than Fe3+ because it has more electrons than Fe3+ and hence the nucleus' inner pull is weaker on it than on Fe3+.
(ii) Which has higher E. A. Fluorine or Neon?
Ans: Fluorine
(iii) Which has maximum metallic character Na, Li or K?
Ans: K
6. Be, Li, C, B, N, O, F (in increasing metallic character)
Si, Na, Al, Mg, Cl, P, S (in decreasing non-metallic character)
Ans: Increasing metallic character: F < O < N < C < B < Be < Li
Decreasing non-metallic character: Cl > S > P > Si > Al > Mg > Na
7. State the trends in chemical reactivity:
(i) Across the period left to right
Ans: The chemical reactivity of elements drops and then increases over time.
(ii) Down the group
Ans: As the potential to lose electrons increases down the group, chemical reactivity increases.
8. A metal M forms an oxide having the formula M2O3. It belongs to the third period. Write the atomic number and valency of the metal.
Ans: The metal is from the third period, and it has three shells.
The compound's chemical formula indicates that the metal's valency is +3. It belongs to the third group since the valence electrons are three.
As a result, the electrical arrangement of the element must be 2, 8, 3.
That indicates there are 13 electrons in all.
Atomic number = 13 Valency = 3 Valency = 3 Valency = 3 Valency = 3 Valency = 3
9. An element X belong to 3rd period and 17th group, state
(i) no of valence electrons in it.
Ans: The outermost shell of an element from the 17th group has 7 electrons.
(ii) Name of the element.
Ans: Chlorine.
(iii) Name the family to which it belongs.
Ans: Halogen family.
(iv) Write the formula of the compound formed when it reacts with 13Y27
Ans: The element's valency is three because it has three electrons in its outermost shell that it can give. While chlorine has a valency of 1, it has a valency of 0. To achieve the stable electrical state, 13Y27, which is Aluminum, can donate three electrons and chlorine can take one electron.
As a result, the compound's formula is AlCl3.
10. The given table shows elements with same number of electrons in its valence shell.
Elements | A | B | C |
M.P | 63.0 | 180.0 | 97.0 |
State:
(i) Whether these elements belong to same group or period.
Ans: Yes, these elements are from the same family, but they aren't from the same time period.
(ii) Arrange them in order of increasing metallic character.
Ans: We know that as we move down the group, m.p. diminishes. As a result of the aforementioned table, the items can be sorted by period as follows:
Elements | B | C | A |
M.P | 180.0 | 97.0 | 63.0 |
As one travels along the group, the metallic character becomes more prominent. As a result, the following is the order of the elements with increasing metallic character:
B < C < A
11. Which one of the following has the largest atomic radius?
i. Sodium
ii. Potassium
iii. Magnesium
iv. Aluminium
Ans: The correct option is second, Potassium.
12. Which one has the largest size?
i. Br
ii. I
iii. I-
iv. Cl
Ans: The correct option is third, I-.
13. The metals of group 2 from top to bottom are Be, Mg, Ca, Sr and Ba.
(i) Which one of these elements will form ions most readily and why?
Ans: The outermost valence electron, which experiences the least force of attraction by a positively charged nucleus, can quickly be given up to form cations, allowing barium to form ions.
(ii) State the common feature in their electronic configuration.
Ans: All elements in Group II contain two valence electrons.
14. Write the number of protons, neutrons, and electronic configuration of 39K19, 31P15, Also state their position in periodic table.
Ans:
39K19
Protons = 19, Neutrons = 39 - 19 = 20
Electronic configuration = 1s2 2s2 2p6 3s2 3p6 4s1
Position in the periodic table = Group 1, Period 4
31P15
Protons = 15, Neutrons = 31 - 15 =16
Electronic configuration = 1s2 2s2 2p6 3s2 3p3
Position in the periodic table = Group 3, Period 3
15. Name the element which has:
(i) two shells, both of which are completely filled with electrons?
Ans: Neon
(ii) the electronic configuration 2, 8, 3?
Ans: Electronic configuration = 2, 8, 3
Therefore, atomic number = 13
The element that has atomic number 13 is Aluminium.
(iii) a total of three shells with five electrons in its valence shell?
Ans: The element has three shells in total, making it a member of the third period. The element belongs to the fifth group if it has five valence electrons (VA). As a result the element is phosphorus.
(iv) a total of four shells with two electrons in its valence shell.
Ans: Calcium
(v) twice as many electrons in its second shell as in its first shell?
Ans: Carbon
Electronic configuration= 1s2 2s2 2P2
Electron in the 1st shell is 2 and the electron in the second shell is 4.
16. The element barium has atomic number 56. Look up its position in the periodic table and answer the following questions:
(i) Is it a metal or a non-metal?
Ans: It is a metal since it belongs to group II and contains two valence electrons.
(ii) Is it more or less reactive than calcium?
Ans: In the group, barium is placed below calcium. Barium is more reactive than calcium because reactivity increases below the group.
(iii) What is its valency?
Ans: Its valency is two because it has to shed two valence electrons to complete its octet configuration.
(iv) What will be the formula of its phosphate?
Ans: Its phosphate formula will be Ba3 (PO4)2.
(v) Is it larger or smaller than caesium (Cs) in size?
Ans: The size drops as we move from left to right in a period, therefore it will be smaller than caesium.
17. In group I of the periodic table, three elements X, Y and Z have ionic radii 1.33 A0, 0.95 A0 and 0.60 A0, respectively. Giving a reason, arrange them in the order of increasing atomic numbers in the group.
Ans: The ionic radii will rise as the size of the atom increases in the group. As a result, the group's growing atomic number order is Z <Y< X.
18. Explain why the following statements are not correct:
(i) All groups contain metal and non-metal.
Ans: Metals and non-metals are not found in any of the groups. Only metals are found in Groups I and II.
(ii) Atoms of elements in the same group have same number of electron(s).
Ans: The number of valence electrons in the atoms of elements in the same group is the same. In their outermost shells, they each have the same number of electrons.
(iii) Non-metallic character decreases across a period with increase in atomic number.
Ans: With an increase in atomic number, the non-metallic nature increases over time. This is due to the fact that the atom's size reduces over time and the valence shell electrons are held more firmly.
(iv) Reactivity increases with atomic number in group as well as in a period.
Ans: The reactivity of elements reduces and then increases as you move from left to right in a period, but chemical reactivity of metals increases as you move down the group, while non-metal reactivity declines.
19. (i) State the number of elements in Period 1, Period 2 and Period 3 of the periodic table. Name them.
Ans: Period 1:
Number of elements = 2
Hydrogen, helium
Period 2:
Number of elements = 8
Lithium, beryllium, boron, carbon, nitrogen, oxygen, fluorine, neon
Period 3:
Number of elements = 8
Sodium, magnesium, aluminium, silicon, phosphorus, sulphur, chlorine, argon
(ii) What is the common feature of the electronic configuration of the elements at the end of Period 2 and Period 3?
Ans: The atoms have 8 electrons in their outermost shell, which is a common property of the electronic configuration of the elements near the conclusion of Period 2 and Period 3.
(iii) If an element is in Group 17, it is likely to be ______ (metallic/non-metallic) in character, while with one electron in its outermost energy level (shell), then it is likely to be _______ (metallic/non-metallic).
Ans: non-metallic, metallic.
(iv) In Period 3, the most metallic element is __________
(sodium / magnesium / aluminium).
Ans: Sodium.
20. Complete the following sentences choosing the correct word or words from those given in brackets at the end of each sentence:
(i) The properties of the elements are a periodic function of their _____ (atomic number, mass number, relative atomic mass).
Ans: atomic number.
(ii) Moving across a _____ of the periodic table, the elements show increasing _____ character (group, period, metallic, non-metallic).
Ans: period, non-metallic character.
(iii) The elements at the bottom of a group would be expected to show _____ metallic character than the element at the top (less, more).
Ans: more
(iv) The similarities in the properties of a group of elements are because they have the same _____ (electronic configuration, number of outer electrons, atomic numbers).
Ans: number of outer electrons.
21. (i) Give reasons for the following:
The size of a Cl- ion is greater than the size of a Cl atom.
Ans: The gain of electrons produces an anion. The number of electrons in the chloride ion is greater than the number of protons. Because the nucleus' effective positive charge is lower, there is less inward attraction. As a result, the size grows.
ii. Argon atom is bigger than chlorine atom.
Ans: After chlorine, the inert gas argon is the next element in the third phase. Due to an increase in nuclear charge with a rise in atomic number, the size of an atom shrinks from left to right over time. Inert gas atoms, on the other hand, are larger than the previous halogen atom in the respective period. Because the outer shell of inert gases is complete, this is the case. They have the most electrons in their outermost orbit, hence electronic repulsions are the strongest. As a result, the atom size of an inert gas is larger.
(iii) Ionisation potential of the element increases across a period.
Ans: The element's ionisation potential increases with time as the atomic size shrinks due to an increase in nuclear charge, requiring more energy to remove the electron (s).
(iv) Inert gases do not form ion.
Ans: Atoms must complete their octet by sharing, losing, or gaining electrons in order to achieve stability. The octet is complete in inert gases, therefore they don't need to gain, lose, or share electrons. As a result, inert gas elements do not produce ions.
Intext Exercise-3
1. (a) Define the term 'ionization potential'.
Ans: Ionization energy or ionisation potential is the amount of energy necessary to remove an electron from a neutral isolated gaseous atom and transform it to a positively charged gaseous ion.
(b) Represent it in the form of an equation. In which unit it is measured?
Ans: M (g) + I.E → M + (g) + e-
M can be any element. It is generally measured in electron volts per atom. It's S.I unit kJmol-1.
2. Ionisation Potential values depend on
a. atomic size
b. nuclear pull. Explain.
Ans: Ionisation potential values depend on
a. Atomic size: The force of attraction decreases as the atomic size increases. The outermost shell electrons are further away from the nucleus, making their removal easier and requiring less ionisation energy.
b. Nuclear charge: The attraction for the electrons in the outermost shell increases as the nuclear charge increases. As a result, the electrons in the outermost shell are more tightly bound, requiring more energy to release them.
3. State the trends in ionization energy:
(a) across the period
(b) down the group
Ans: (a) As the atomic size lowers, the ionisation energy increases as we move from left to right throughout a period.
(b) As the atomic size grows larger, the ionisation energy drops.
4. Name the elements with highest and lowest ionization energies in first three periods.
Ans: In the first three periods, helium has the highest ionisation energy of all the elements, while sodium has the lowest.
5. Arrange the elements of second and third period in increasing order of ionization energy.
Ans: Second period: Lithium < Beryllium < Boron < Carbon < Nitrogen < Oxygen < Fluorine < Neon
Third Period: Sodium < Magnesium < Aluminum < Silicon < Phosphorus < Sulphur < Chlorine < Argon
6. (a) Define the term electron affinity.
Ans: Electron affinity is the energy released when a neutral gaseous atom acquires an electron to form an anion.
(b) Arrange the elements of second period in increasing order of electron affinity. Name the elements which do not follow the trend in this period.
Ans: Second period: Lithium < Boron < Carbon < Oxygen < Fluorine.
Neon, Nitrogen and Beryllium do not follow the trend.
7. Electron affinity values generally ----- across the period left to right and ------down the group top to bottom.
Ans: increase, decrease
8. (a) Define the term 'Electronegativity'. State its unit.
Ans: Electronegativity is the tendency of an atom in a molecule to attract the shared pair of electrons towards itself. Electronegativity is a dimensionless property; therefore, it has no unit.
(b) Among the elements given below, the element with least electronegativity is
(i) Lithium, (ii) Boron, (iii) Carbon, (iv) Fluorine
Ans: The correct option is (i).
(c) The most electronegative element from the following element is:
(i) Magnesium (ii) Chlorine
(iii) Aluminium (iv) Sulphur
Ans: The correct option is (ii).
9. Explain the following:
(a) Group 17 elements are strong non-metals, while group I elements are strong metals.
Ans: Nuclear pull increases as the atomic number grows, and consequently the atomic size shrinks as the period progresses. As a result, elements cannot easily shed electrons. As a result, strong non-metals make up Group 17, while strong metals make up Group 1.
(b) Metallic character of elements decreases from left to right in a period while it increases in moving down a group.
Ans: Nuclear pull increases as the atomic number grows, and consequently the atomic size shrinks as the period progresses. As a result, elements cannot easily shed electrons. As a result, strong non-metals make up Group 17, while strong metals make up Group 1. The atomic size and nuclear charge both grow as you move down a group. When compared to increased nuclear charge, the effect of increased atomic size is larger. As a result, metallic nature rises as one proceeds down a group, implying that electrons can be easily lost.
(c) Halogens have a high electron affinity.
Ans: Halogens have an extremely short atomic size. The greater the electron affinity, the smaller the atomic size, because the effective attractive force between the nucleus and the valence electrons is greater in smaller atoms, holding the electrons securely in place.
(d) The reducing power of element increases down in the group while decreases in a period.
Ans: The reducing property is determined by the elements' ionisation potential and electron affinity. The electron affinity and ionisation energy both rise when the atomic size drops and the nuclear charge increases in a period, from left to right in a horizontal row of the periodic table. As a result, the tendency to lose electrons reduces as the period progresses from left to right, and the reducing characteristic lowers as well. The electron affinity and ionisation potential drop from top to bottom as the group progresses. As a result, the tendency to lose electrons increases, and the reducing characteristic increases as well, from top to bottom in the group.
(e) Size of atoms progressively becomes smaller when we move from sodium (Na) to chlorine (Cl) in the third period of the periodic Table.
Ans: An atom's size falls from left to right during a period. This is due to the fact that the nuclear charge, or atomic number, grows from left to right with time, bringing the outermost shell closer to the nucleus. As a result, when the third period is taken into account, sodium is determined to be the largest, while chlorine is the lowest.
10. Name the periodic property which relates to the:
(i) Amount of energy required to remove an electron from an isolated gaseous atom.
Ans: Ionization energy
(ii) Character of element which loses one or more electrons when supplied with energy.
Ans: Metallic character
(iii) Tendency of an atom to attract the shared pair of electrons.
Ans: Electronegativity
11. This question refers to the elements of the periodic table with atomic numbers from 3 to 18. Some of the elements are shown by letters, but the letters are not the usual symbols of the elements.
3 4 5 6 7 8 9 10
A B C D E F G H
11 12 13 14 15 16 17 18
I J K L M N O P
Which of these:
(i) Are the most electronegative element.
Ans: G due to the smallest atomic size.
(ii) Is a halogen.
Ans: G and O because both have np5 as their outermost electronic configuration.
(iii) Is an alkali metal.
Ans: A and I because both have ns1 as their outermost electronic configuration
(iv) Is an element with valency 4.
Ans: D (1s2 2s2 2p2).
(v) Have least ionisation energy.
Ans: Ionisation energy is lowest in alkali metals. Also, when the atomic size grows larger, the ionisation energy lowers, and as the group size decreases, the ionisation energy decreases.
(vi) have least atomic size in Period 3
Ans: O, the atomic size of halogens is the smallest.
12. A group of elements in the Periodic Table are given below (boron is the first member of the group and thallium is the last).
Boron, Aluminium, Gallium, Indium, Thallium
Answer the following questions in relation to the above group of elements:
(a) Which element has the most metallic character?
Ans: Thallium, thallium will have the greatest metallic character because the metallic character increases down the group.
(b) Which element would be expected to have the highest electronegativity?
Ans: Boron, as the size of the group diminishes, electronegativity falls, and boron will be the most electronegative atom.
(c) If the electronic configuration of aluminium is 2, 8, 3, how many electrons are there in the outer shell of thallium?
Ans: Three, for each group, the number of electrons in the valence shell is the same. As a result, all of these elements, with the exception of thallium, will have three valence electrons.
(d) The atomic number of boron is 5. Write the chemical formula of the compound formed when boron reacts with chlorine.
Ans: BCl3
(e) Will the elements in the group to the right of this boron group be more metallic or less metallic in character? Justify your answer.
Ans: Because metallic character declines as one moves from left to right and non-metallic character increases, elements in the group to the right of this boron group will be less metallic.
Exercise-1
1. What is the significance of atomic number in modern periodic table?
Ans: The periodic chart arranges elements in increasing order of their atomic number. It claims that element qualities are periodic functions of their atomic number, i.e., if the elements are placed in tabular form in ascending order of their atomic numbers, the properties of the elements are repeated at fixed regular intervals or periods.
2. Arrange the following as per instructions given in the brackets.
(i) Mg, Cl, Na, S, Si (increasing order of atomic size).
Ans: Cl < S < Si < Mg < Na
(ii) Cs, Na, Li, K, Rb (increasing metallic character)
Ans: Li < Na < K < Rb < Cs
(iii) Na, K, Cl, S, Si (increasing ionisation potential)
Ans: Cl < S < Si < Na < K
(iv) Cl, F, Br, I (increasing electron affinity)
Ans: I < Br < F < Cl
(v) Cs, Na, Li, K, Rb (decreasing electronegativity)
Ans: Li > Na > K = Rb > Cs
(vi) K, Pb, Ca, Zn (increasing reactivity)
Ans: Pb < Zn < Ca < K
(vii) Li, K, Na, H (decreasing order of their potential ionisation)
Ans: H > Li > Na > K
3. Arrange the following as per instructions given in the brackets.
(i) Mg, Cl, Na, S, Si (increasing order of atomic size)
Ans: Cl < S < Si < Mg < Na
(ii) Cs, Na, Li, K, Rb (increasing metallic character)
Ans: Li < Na < K < Rb < Cs
(iii) Na, K, Cl, S, Si (increasing ionisation potential)
Ans: Cl < S < Si < Na < K
(iv) Cl, F, Br, I (increasing electron affinity)
Ans: I < Br < F < Cl
(v) Cs, Na, Li, K, Rb (decreasing electronegativity)
Ans: Li > Na > K = Rb > Cs
1.0 > 0.9 > 0.8 = 0.8 > 0.7
4. First Ionization enthalpy of two elements X and Y are 500 kJ/mol-1 and 375 kJ /mol-1 respectively. Comment about their relative position in a group as well in a period.
Ans: The ionisation energy is the lowest amount of energy required to remove the outermost electron from a gaseous neutral atom, resulting in the formation of a cation.
Position in a group: Because ionisation energy diminishes as one moves along the group, X will be placed above Y.
Position in a period: Because ionisation energy grows from left to right, X will be on the right side of Y.
5. Arrange the following in order of increasing radii:
(a) Cl¯, Cl
Ans: Cl < Cl¯
(b) Mg2+, Mg, Mg+
Ans: Mg2+ < Mg+ < Mg
(c) N, O, P
Ans: O < N < P
6. Which element from the following has the highest ionization energy?
(a) P, Na, Cl
Ans: Cl
Metals have a low ionisation energy, while non-metals have a high one. In addition, ionisation energy tends to rise over time. The third phase includes the elements P, Na, and Cl. Group 1 is Na, Group 15 is P, and Group 17 is Cl.
(b) F, O, Ne
Ans: Ne
Because of their steady electronic configuration, inert gases have zero electron affinity.
(c) Ne, He, Ar
Explain your choice.
Ans: He
The ionisation energy drops as the atomic size decreases, i.e. as one progresses down a group. Inert gases include Ne, He, and Ar. Period 1 is He, Period 2 is Ne, and Period 3 is Ar.
7. The electronegativity’s (according to Pauling) of the elements in period 3 of the periodic table are as follows with elements arranged in alphabetical order:
Al | Cl | Mg | Na | P | S | Si |
1.5 | 3 | 1.2 | 0.9 | 2.1 | 2.5 | 1.8 |
Arrange the elements in the order in which they occur in the periodic table from left to right.
(The group 1 element first, followed by the group 2 element and so on, up to group 7).
Ans: Na, Mg, Al, Si, P, S, Cl
8. Choose the word or phrase from the brackets which correctly completes each of the following statements:
A. The element below sodium in the same group would be expected to have a ............... (lower/higher) electro-negativity than sodium, and the element above chlorine would be expected to have a (lower/higher) ionisation potential than chlorine.
Ans: lower, higher.
B. On moving from left to right in a given period, the number of shells ................. (remains the same/increases decreases).
Ans: remains the same.
C. On moving down a group, the number of valence electrons .................. (remains the same/increases/decreases).
Ans: the same.
D. Metals are good ................. (oxidising agents/reducing agents) because they are electron ............. (acceptors/donors).
Ans: reducing agents , donors.
9. Parts (a) to (e) refer to change in the properties of elements on moving from left to right across a period of the periodic table. For each property, choose the correct answer.
(a) The non-metallic character of the elements:
(i) Decreases
(ii) Increases
(iii) Remains the same
(iv) Depends on the period
Ans: Increases
(b) The electronegativity:
(i) Depends on the number of valence electrons
(ii) Remains the same
(iii) Decreases
(iv) Increases
Ans: Increases
(c) The ionization potential:
(i) goes up and down
(ii) Decreases
(iii) Increases
(iv) Remains the same
Ans: Increases
(d) The atomic size:
(i) Decreases
(ii) Increases
(iii) Remains the same
(iv) Sometimes increases and sometimes decreases
Ans: Decreases
(e) The electron affinity of elements in group 1 to 7:
(i) Goes up and then down
(ii) Decreases and then increases
(iii) Increases
(iv) Decreases
Ans: Increases
10. The elements of one short period of the periodic table are given below in order from left to right:
Li Be B C O F Ne
(a) To which period do these elements belong?
Ans: Period 2
(b) One element of this period is missing. Which is the missing element and where should it be placed?
Ans: Nitrogen (N), between carbon and oxygen
(c) Place the three elements: Fluorine, Beryllium and nitrogen in the order of increasing electronegativity.
Ans: Be< N< F
(d) Which one of the above element belongs to the halogen series?
Ans: Fluorine
11. With reference to the variation of properties in the Periodic table, which of the following is generally true?
A. Atomic size increases from left to right across a period.
B. Ionization potential increases from left to right across a period.
C. Electron affinity increases going down a group.
D. Electro-negativity increases going down a group.
Ans: B. Electrons are added to the same valence shell as we move from left to right in a period. As a result, atomic size shrinks and nuclear charge rises. The energy required to remove an electron grows as nuclear charge increases, and hence the ionisation potential increases over time.
12. Atomic numbers of elements A, B, C, D, E, F are 8, 7, 11, 12, 13 and 9 respectively. State the type of ions they form.
Ans: These substances will combine to generate anions. These elements belong to the VIA (16), VA(15), IA(1), IIA(2), IIIA(13), and VIIA(17) families, and they form anion in general.
13. (a) Formula of ion of A is A2+. Element A probably belongs to …….. Group.
Ans: Second
(b) In a period, increase in electron affinity increases …………
(oxidation/reduction).
Ans: reduction
(c) On descending a group,……… (increase/decrease) in ionization potential as well as electron affinity …….. (increase/decrease) oxidizing capacity.
Ans: increase, decreases
2009
In the table below, H does not represent hydrogen. Some elements are given in their own symbol and position in the periodic table while others are shown with a letter.
1. (a) Among Period 2 elements A, B, C and D, the one which has high electron affinity is
A. Lithium
B. Carbon
C. Fluorine
D. Neon
Ans: The electron affinity of lithium diminishes as the non-metallic character grows from left to right.
(b)
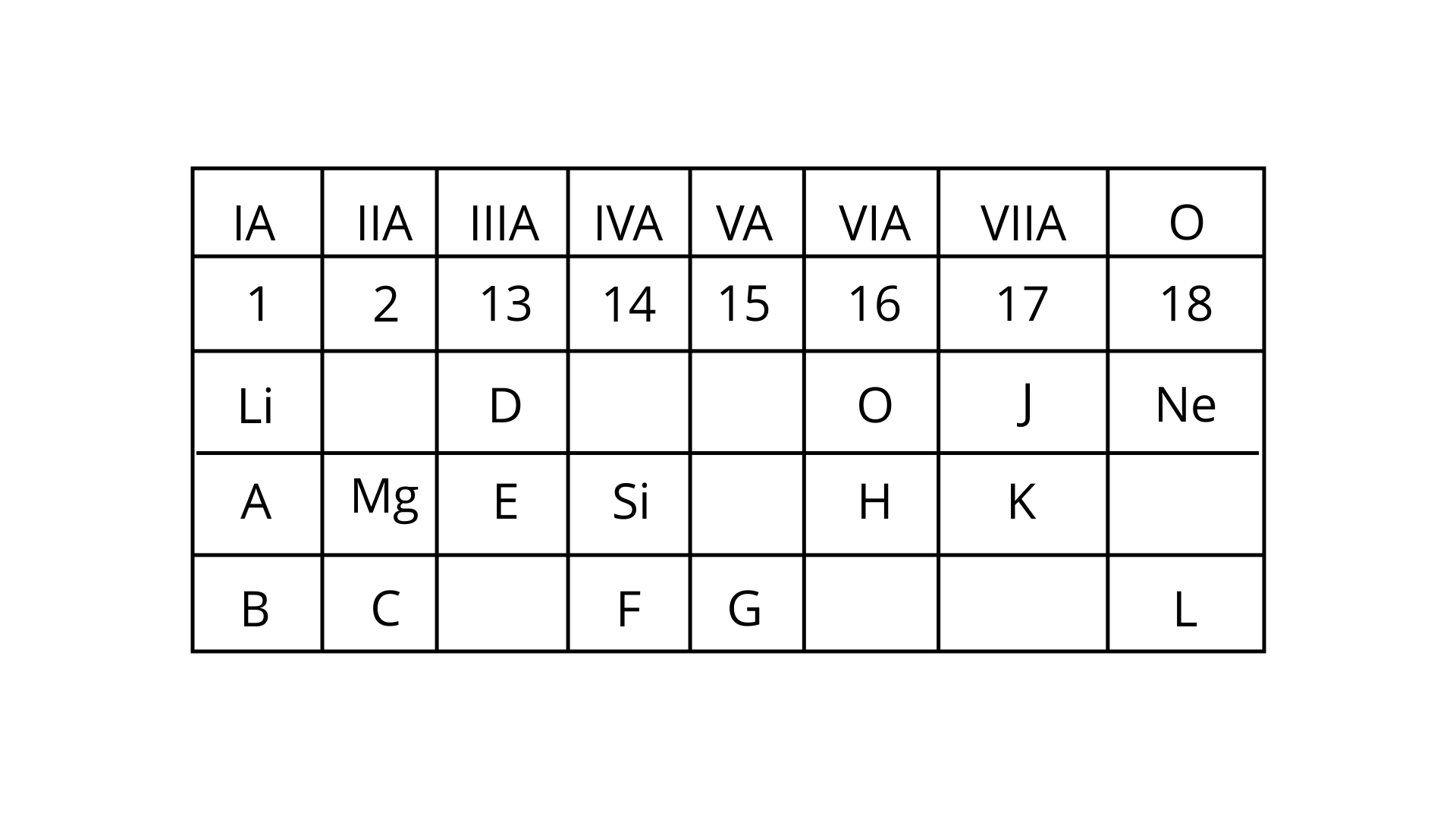
Select from the table:
(i) Which is the most electronegative?
(ii) How many valence electrons are present in G?
(iii) Write the formula of the compound between B and H.
(iv) In the compound between F and J, what type of bond will be formed?
(v) Draw the electron dot structure for the compound formed between C and K.
Ans:
(i) The most electronegative is J.
(ii) Valence electrons present in G are 5.
(iii) B has one valence electron, while H has six valence electrons. As a result, B has a valency of +1 while H has a valency of -2.
(iv)The type of bond created in the compound between F and J will be covalent.
(v)
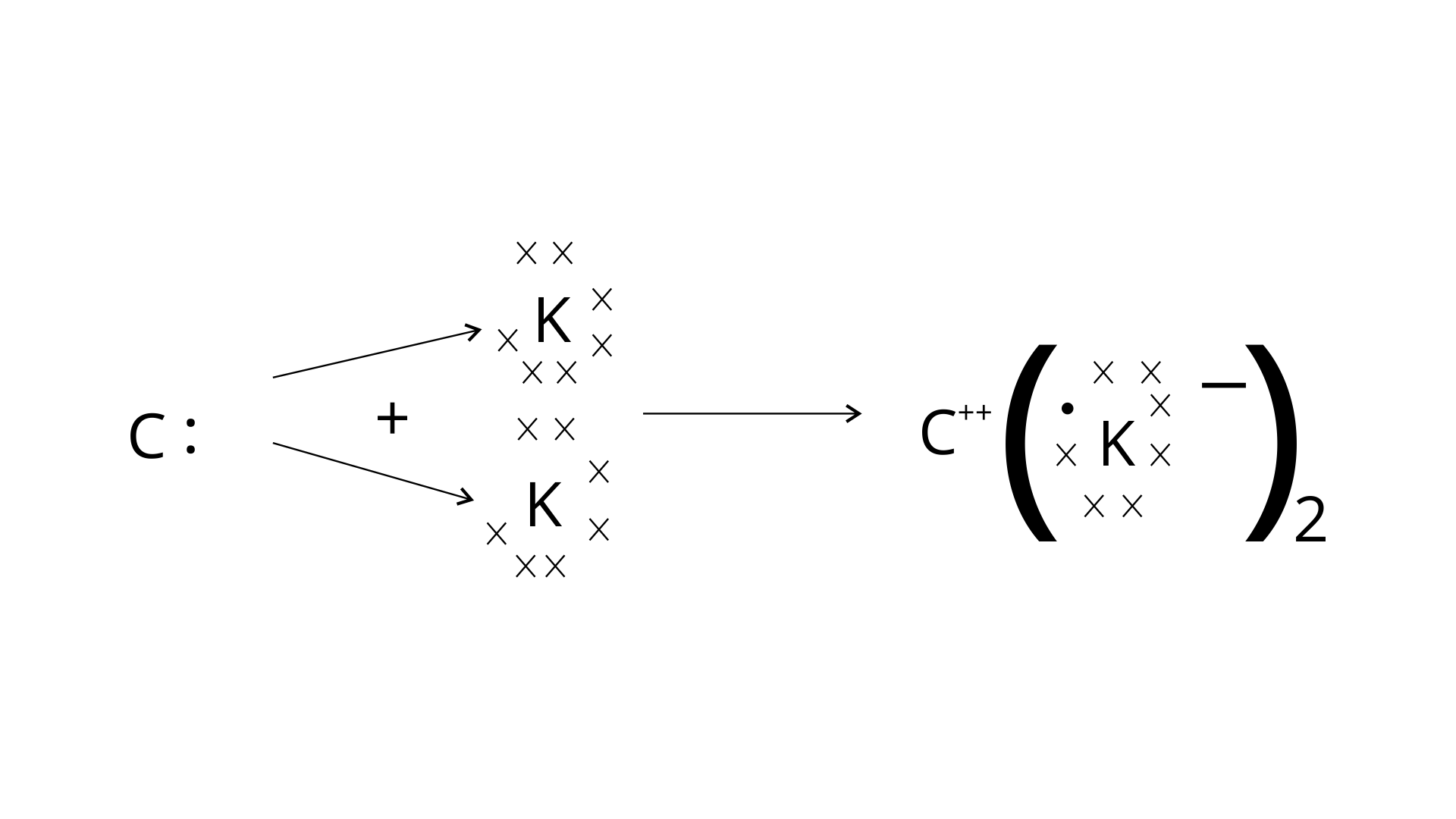
2010
(a) The number of electrons in the valence shell of a halogen is ……….
A - 1
B - 3
C - 5
D - 7.
Ans: The number of electrons in the valence shell of a halogen is 7.
(b) Electronegativity across the period ............ (increases/decreases).
Ans: Electronegativity across the period increases.
(c) Non-metallic character down the group ………… (increases/decreases).
Ans: Non-metallic character down the group decreases.
(d) Atomic number of an element is 16. State
(i) to which period it belongs.
Ans: It belongs to Period 3.
(ii) the number of valence electrons in the element.
Ans: The number of valence electrons in the element is 6.
(iii) is the element metal or non-metal
Ans: The element is a non-metal.
2011
(a) Give reasons - The oxidising power of elements increases from left to right along a period.
Ans: The oxidising power of elements is determined by their tendency to acquire electrons, which rises from left to right over time as nuclear pull increases.
(b) Select the correct answer:
(i) Across a period, the ionisation potential ………… (increases, decreases, remains same)
Ans: increases.
(ii) Down the group, electron affinity ………… (increases, decreases, remains same)
Ans: decreases.
(c) Choose the correct answer from the choice given:
(i) In the periodic table, alkali metals are placed in the group
A : 1
B : 11
C : 17
D : 18.
Ans: The correct option is A.
(ii) Which of the following properties do not match with elements of the halogen family?
A. They have seven electrons in their valence shell.
B. They are highly reactive chemically.
C. They are metallic in nature.
D. They are diatomic in their molecular form.
Ans: The correct option is C.
(d) State the group and period of the element having three shells with three electrons in the valence shell.
Ans: The presence of three shells indicates that the piece is from the third period. The element belongs to the third group if it has three valence electrons.
2012
(a) Choose the correct answer from the option: An element in Period 3 whose electron affinity is zero.
A. Neon
B. Sulphur
C. Sodium
D. Argon
Ans: The correct option is (D).
(b) Give reason:
(i) Ionisation potential of the element increases across a period from left to right.
Ans: Because the atomic radius diminishes over time. As a result, the nucleus and electron attract each other more strongly. The ionisation potential rises as a result of this.
(ii) Alkali metals are good reducing agents.
Ans: Because they have a higher tendency to lose electrons, alkali metals are good reducing agents.
(c) There are three elements E, F, G with atomic numbers 19, 8 and 17 respectively-
Classify the above elements as metals and non-metals.
Ans: Electronic configuration of E with atomic number 19 = 1s2 2s2 2p6 3s2 3p6 4s1
E is a metal.
Electronic configuration of F with atomic number 8 = 1s2 2s2 2p4
F is a non-metal.
Electronic configuration of G with atomic number 17 = 1s2 2s2 2p6 3s2 3p5
G is non-metal.
(d) Name: A metal present in Period 3, Group I of the periodic table.
Ans: Sodium is a metal that belongs to Period 3, Group I of the periodic table.
2013
(a) Among the Period 2 elements, the element which has high electron affinity is
A. Lithium
B. Carbon
C. Chlorine
D. Fluorine
Ans: The correct option is (D).
(b) In the table below, H does not represent hydrogen. Some elements are given in their own symbol and position in the periodic table while others are shown with a letter.
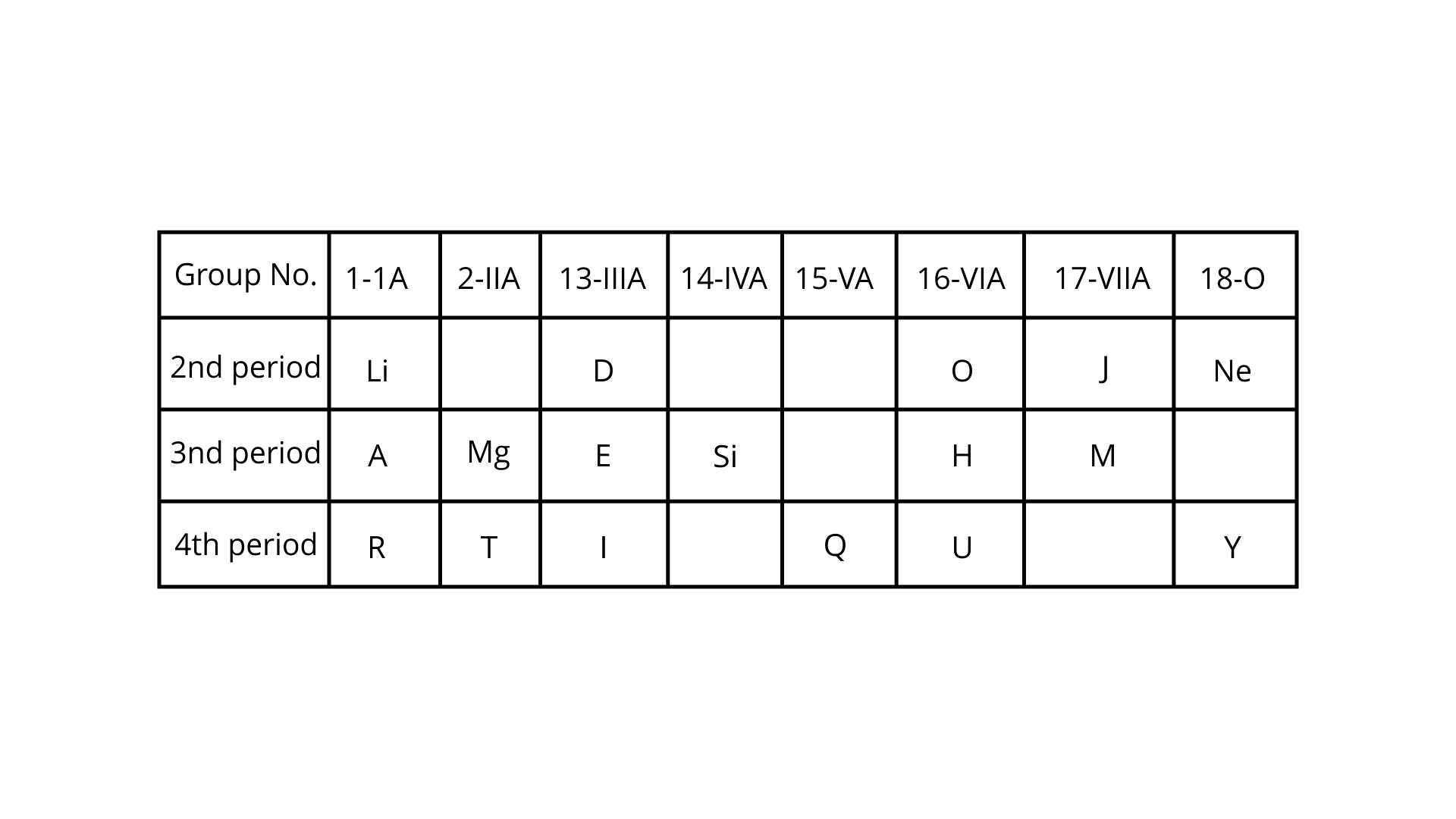
Answer the following questions.
(i) Identify the most electronegative element.
Ans: I
(ii) Identify the most reactive element of Group I.
Ans: R
(iii) Identify the element from Period 3 with least atomic size.
Ans: M
(iv) How many valence electrons are present in Q?
Ans: 5
(v) Which element from group 2 would have the least ionisation energy?
Ans: T
(vi) Identify the noble gas of the fourth period.
Ans: Y
(vii) In the compound between A and H, what type of bond would be formed and give its molecular formula.
Ans: The chemical formula is A2H, and ionic bonds will form.
(c) Identify: The element which has the highest ionisation potential.
Ans: Helium is the element with the highest ionisation potential (He).
2014
Choose the correct answer from the choice given:
i. Ionisation potential increases over a period from left to right because the:
A. Atomic radius and nuclear charge increase
B. Atomic radius and nuclear charge decrease
C. Atomic radius increases and nuclear charge decreases
D. Atomic radius decreases and nuclear charge increases
Ans: D
ii. An element A belonging to Period 3 and Group II will have
A. 3 shells and 2 valence electrons
B. 2 shells and 3 valence electrons
C. 3 shells and 3 valence electrons
D. 2 shells and 2 valence electrons
Ans: A
(b) An atomic number of an element Z is 16. Answer the following:
(i) State the period and group to which Z belongs.
Ans: Sulphur belongs to Period 3 and Group 16.
(ii) Is Z a metal or a non-metal?
Ans: Sulphur is a non-metal.
(c) State the formula of the compound between Z and hydrogen.
Ans: Hydrogen sulphide (H2S) is formed when two hydrogen atoms interact with one sulphur atom.
(d) M is a metal above hydrogen in the activity series and its oxide has the formula M2O. This oxide, when dissolved in water, forms the corresponding hydroxide which is a good conductor of electricity. In the above context, answer the following:
(i) What kind of combination exists between M and O?
Ans: Ionic bond exists between M and O.
(ii) State the number of electrons in the outermost shell of M.
Ans: 1 electron exists in the outermost shell of M.
(iii) Name the group to which M belongs.
Ans: M belongs to Group 1 in the periodic table.
(e) Give one word or phrase for: The amount of energy released when an atom in the gaseous state accepts an electron to form an anion.
Ans: Electron affinity
(f) Match the option A and B with the statements (i) and (ii):
Metal | (i) The metal that forms two types of ions |
Iron | (ii) An element with electronic configuration 2,8,8,3 |
Ans:
A-(ii)
B-(i)
2015
(a) Among the elements given below, the element with the least electronegativity is:
(i) Lithium
(ii) Carbon
(iii) Boron
(iv) Fluorine
Ans: Lithium, From left to right, electronegativity rises. Lithium is the least electronegative element because it is found on the left side of the periodic table.
(b) The metals of Group 2 from top to bottom are Be, Mg, Ca, Sr and Ba.
(i) Which of these elements will form ions most readily and why?
Ans: Because the ionisation energy lowers as the group size grows, Ba metal quickly forms ions.
(ii) State the common feature in the electronic configuration of all these elements.
Ans: The number of electrons in the outermost shell, i.e. valence electrons, remains constant as you move down the group. As a result, the valency of a group remains constant, i.e. 2.
2016
(a) Metals are good _____________ (oxidising agents/reducing agents) because they are electron __________ (acceptors/donors).
Ans: reducing agents, donors.
(b) An element with the atomic number 19 will most likely combine chemically with the element whose atomic number is:
A. 17
B. 11
C. 18
D. 20
Ans: 17, to reach the noble gas configuration, an element with atomic number 19 will lose one electron, which the element with atomic number 17 can absorb.
(c) Rewrite the following sentences by using the correct symbol > (greater than) or < (less than) in the blanks given:
1. The ionization potential of potassium is _________________ that of sodium.
Ans: less than
2. The electronegativity of lodine is ___________ that of Chlorine.
Ans: less than
(d) Fill in the blanks by selecting the correct word from the brackets:
i. If an element has a low ionization energy then it is likely to be ______________ (metallic / non-metallic).
Ans: Metallic
ii. If an element has seven electrons in its outermost shell then it is likely to have the ______________ (largest / smallest) atomic size among all the elements in the same period.
Ans: Smallest
2018
(a) In Period 3 of the Periodic table, element B is placed to the left of element A. On the basis of this information, choose the correct word from the brackets to complete the following statements:
(i) The element B would have (lower /higher) metallic character than A.
Ans: The element B would have a higher metallic character than A.
(ii) The element A would probably have (lesser / higher) electron affinity than B.
Ans: The element A would probably have higher electron affinity than B.
(iii) The element A would have (greater /smaller) atomic size than B. Ans: The element A would have a smaller atomic size than B.
2019
Study the extract of the periodic table given below and answer the questions that follow. Give the letter corresponding to the element in question. DO NOT repeat an element.

(i) Which element forms an electrovalent compound with G?
(ii) The ion of which element will migrate towards the cathode during electrolysis?
(iii) Which non-metallic element has the valency of 2?
(iv) Which is an inert gas?
Ans:
(i) Element B forms an ionic compound with G.
(ii) The B2+ will move towards the cathode during the electrolysis process.
(iii) The non-metallic element which has the valency of 2 is E.
(iv) F is an inert gas.
What Exactly is the Periodic Table?
The periodic table is an arrangement of all known elements in order of increasing atomic number and recurring chemical properties. They are organized in a tabular format, with a row representing a period and a column representing a group.
Elements are arranged in increasing atomic number order from left to right and top to bottom. Thus, Elements in the same group will have the same valence electron configuration and, as a result, will have similar chemical properties.
Elements in the same period, on the other hand, will have an increasing order of valence electrons. As a result, as the atom's energy level rises, so does the number of energy sublevels per energy level.
The first 94 elements of the periodic table are naturally occurring, while the remaining elements from 95 to 118 have been synthesized.
The modern periodic table, which we now use, is a new and improved version of certain models proposed by scientists in the nineteenth and twentieth centuries. Dimitri Mendeleev proposed his periodic table based on the discoveries of previous scientists such as John Newlands and Antoine-Laurent de Lavoisier. Mendeleev, on the other hand, is given sole credit for developing the periodic table.
Periodic Table of Mendeleev
The first iteration of the periodic table, which is still in use today, was proposed by Dimitri Mendeleev, widely regarded as the father of the periodic table. Mendeleev's periodic law differs from modern periodic law in one important way.
Mendeleev based his periodic table on increasing atomic mass, whereas modern periodic law is based on increasing atomic number order.
Mendeleev's periodic table, despite being based on atomic weight, was able to predict the discovery and properties of certain elements. During his time, only about half of the elements now known to us were known, and most of the information we had on the elements was incorrect. Mendeleev's Periodic Table was first published in 1869 in the German Journal of Chemistry.
The elements in the periodic table are listed in increasing atomic number order. All of these elements exhibit several other trends, and we can predict their chemical, physical, and atomic properties using the periodic law and table formation. Understanding these trends requires looking at the electron configuration of the elements; all elements prefer an octet formation and will gain or lose electrons to achieve that stable configuration.
The Atomic Radius
We can never determine an atom's atomic radius because there is never a zero probability of finding an electron, and thus no distinct boundary to the atom. We can only measure the distance between two nuclei (internuclear distance). A covalent radius is one-half the distance between two identical atoms' nuclei. In an ionic bond, an ionic radius is one-half the distance between the nuclei of two ions. The distance must be divided between the smaller cation and the larger anion. In a crystalline structure, a metallic radius is one-half the distance between the nuclei of two adjacent atoms. Because the experimental values of the noble gases' atomic radii are highly debated, they are excluded from the trends in atomic radii. The nanometer (nm) and picometer (pc) are the SI units for measuring atomic radii (pm).
Electron Affinity
The energy change that occurs when an electron is added to a gaseous atom is referred to as electron affinity (E.A.). Electron affinity is also defined as the enthalpy change caused by the addition of an electron to a gaseous atom. It can be either positive or negative. The more negative the value, the more stable the anion.
Electronegativity
The ability of an atom to compete for electrons in a bond is measured as electronegativity. The higher the electronegativity, the greater the bond's ability to gain electrons. Electronegativity will be important in determining polar and nonpolar molecules later on. Ionization energy and electron affinity are related to electronegativity. Because their nuclei do not exert a strong attractive force on electrons, electrons with low ionization energies have low electronegativities. Because of the strong pull of the positive nucleus on the negative electrons, elements with high ionization energies have high electronegativities. As a result, electronegativity rises from bottom to top and from left to right.
Uses for Learning About the Periodic Properties of Elements
Predicting atomic size and radial distribution in neutral atoms and ions.
Ionization energies are measured and compared.
Electron affinities and electronegativities are compared.
estimating redox potential
When compared to other elements, the metallic character is distinguished by its ability to form cations.
Predicting what reaction might or might not occur as a result of the trends
Choosing the reaction with the greater cell potential (the sum of oxidation and reduction potential)
Completing chemical reactions based on trends
Selina Concise ICSE Solutions for Class 10 Chemistry Chapter 1 Periodic Table, Periodic Properties, and Variations of Properties are provided by Vedantu in step-by-step solutions. Selina Concise Chemistry ICSE Solutions for Class 10 are available for free PDF download. All questions in Selina Publishers Concise Chemistry for Class 10 ICSE Solutions are solved and explained by expert teachers in accordance with ICSE board guidelines.
FAQs on Periodic Table, Periodic Properties and Variations of Properties Solutions for Class 10 Chemistry
1. Do I need to practice all the questions provided in Variations of Properties Solutions for Class 10 Chemistry?
Yes, you need to practice all the questions provided in Variations of Properties Solutions for Class 10 Chemistry. It helps you to score better in your board examinations. Selina Publishers give you a step-by-step guide for all the solutions which will help you to understand all the concepts better. Your doubt can be clarified on your own self-study when you refer to Selina Publishers. Periodic table and its properties being a very important topic in the Chemistry of class 10, it is important you know the atomic numbers of all the elements which will help you to solve many numeric problems from the chapter. That will give you an edge in scoring Top marks in the Board Examinations.
2. How can I understand the class 10 Chemistry Periodic Table and Properties?
Selina Solutions will help you to understand the class 10 Chemistry Periodic Table and Properties. Vedantu helps you to understand all the concepts better with the Pdf solutions of Selina solutions provided above. You just have to log in with your mobile number and get registered on Vedantu.com, you can download the pdf solutions from there. Vedantu is a student-friendly app. Master class faculty of Vedantu have curated their knowledge and helped students to make their concepts better.
3. What is the definition of the periodic table of elements according to class 10 Chemistry?
In Chemistry, a periodic table is an organized array of all the chemical elements in order of increasing atomic number—that is, the total number of protons in the atomic nucleus. When the chemical elements are arranged in this manner, there is a recurring pattern in their properties known as the "periodic law," in which elements in the same column (group) have similar properties. The initial discovery, made by Dmitry I. Mendeleyev in the mid-nineteenth century, has been of incalculable value in the advancement of Chemistry.
4. Where can I get the Periodic Table Free Download for class 10 Chemistry?
You can get the Periodic Table Free Download for class 10 Chemistry in the Vedantu site or app. Vedantu provides Selina Solutions for Periodic Table and its properties which are very easy to understand. They are solved in the step-by-step process which will help the students study on their own. Vedantu Revision notes also help you to understand each concept in the easiest way. And these study materials are completely free and easy to download. These resources will be an added advantage for your preparation.
5. How to score well in Chemistry in class 10?
The following are the important tips to score well in Chemistry:
1. Read through the NCERT books from beginning to end.
2. Pay special attention to the units with the highest weightage.
3. Examine previous years' question papers
4. Memorize the Physical Chemistry formulas
5. Practice Organic and Inorganic Reasoning Questions
6. For Inorganic Chemistry, focus on the p-block elements and Coordination Compounds chapters.
7. It is critical to have named reactions and conversions.
8. Experiment with IUPAC naming and compound identification using various agents.
9. The last three chapters are worth a total of ten points.









































