




Chemical Reactions of Metals with Oxygen Explained
Air and a variety of metals react to generate metal oxides. Sodium and potassium are both soft metals that are easily sliced, exhibiting a surface that is initially shiny but quickly becomes dull.
The volatile oxides evaporation is created when platinum reacts with air at high temperatures, which significantly raises the platinum losses in oxygen-containing environments when compared to vacuum situations.
Due to their strong reactions with air, lithium, potassium, and sodium are kept submerged in kerosene oil to avoid any unintended reactions with either air or water. The nature of the metal oxides is basic. The basic metal oxides shift red litmus paper to blue. So, in this article let’s see more about the reactivity of metals with air using certain examples with its reactions.
What Happens When Metals are Burnt in Air?
Metal oxides are created when specific metals burnt in air.
\[\text{Metals}+ \text{Oxygen}\to \text{MetalOxide}\]
The majority of metals have this chemical feature, combining with oxygen to create the corresponding metallic oxides.
The examples of what happens when metals are burnt in air are explained below:
What Happens When Magnesium is Burnt in Air?
Magnesium Mg burns in air to give magnesium oxide (MgO) and while burning it appears a dazzling light.
\[2Mg + {O_2} \to 2MgO\]
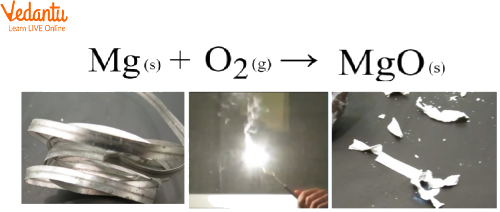
Mg Burnt in Air
What Occurs if Sodium Burns in the Air?
If sodium is heated in the presence of air, it begins to burn with an orange glow and produces a combination of sodium peroxide (Na2O2) and sodium oxide (Na2O).
If sodium is heated or exposed to flames, it begins to burn in the air right away.
\[4Na + {O_2} \to 2N{a_2}O\]
\[2Na + {O_2} \to N{a_2}{O_2}\]
What Occurs When Zinc Burns in the Air?
When burned in air, zinc initially begins to vaporize before a light coating of zinc oxide is created on top of it.
\[2Zn + {O_2} \to 2ZnO\]
What Occurs When Iron Burns in the Air?
Iron filings burn strongly when exposed to air and are sprayed with burner flame. Just when iron is burnt in the air, iron gets hot. Iron doesn't react with oxygen in the air and doesn't even react when heated.
What Occurs When Copper (Cu) Burns in the Air?
Whenever copper is heated or burned in the presence of atmospheric air, a coating of copper (II) oxide (CuO) that is black in colour forms on top of the metal.
\[2Cu + {O_2} \to 2CuO\]
Although copper (II) oxide forms a dark layer on top of it, it does not burn in air. Copper (I) oxide (Cu2O) is also formed through the oxidation of copper and copper peroxide (CuO2), and copper (III) oxide (Cu2O3) also exists.
What Occurs When Lithium Burns in the Air?
Lithium begins to burn with a red-tinged flame whenever heated or burned in the presence of air, producing lithium oxide.
When heated or exposed to flame, lithium also instantly begins to burn with a red-tinged flame.
Effects of Burning Potassium in Air
Potassium produces potassium superoxide (KO2) and potassium peroxide (K2O2) whenever it is burned in the air. Potassium can also form potassium oxide (K2O) when burnt in atmospheric air.
\[2K + {O_2} \to {K_2}{O_2}\]
Whenever potassium is heated or exposed to flames, it dissolves instantly.
Interesting Facts
Since some metals are quickly oxidised, the metals near the top of the reactivity series are strong reducing agents. These metals rust/tarnish quite quickly.
As you move down the series, the metals' reducing power becomes less and less effective.
While descending the metal reactivity sequence, the electropositivity of the metals similarly decreases.
When interacting with diluted HCl or H2SO4, all metals above hydrogen in the reactivity sequence release H2 gas.
Higher ranking metals demand more energy to separate them from ores and other molecules.
Key Features to Remember
Metal oxide is created when a metal and air reacts together.
Metal + oxygen = metal oxide is the fundamental equation for the metal reaction with air.
When iron is subjected to air, a type of iron oxide called rust is formed slowly. Steel, an alloy that can be created from iron, has a higher corrosion resistance.
When iron metal is in contact with heat from a burner and subjected to air, iron filings burn vigorously.
It does not ignite in air despite copper oxide building up a black coating on top of it.
FAQs on What Happens When Metals Are Burnt in Air?
1. What generally happens when metals are burnt in air? Explain with a common example.
When most metals are burnt in air, they undergo a rapid oxidation reaction with the oxygen present. This chemical reaction results in the formation of a new compound called a metal oxide. For example, when a magnesium ribbon is burnt, it reacts with oxygen to produce a dazzling white light and a white, powdery ash of magnesium oxide (MgO).
The general equation for this reaction is: Metal + Oxygen → Metal Oxide.
2. What are amphoteric oxides? Give an example of a metal that forms one when it reacts with air.
Amphoteric oxides are metallic oxides that exhibit both acidic and basic properties. This means they can react with both acids and bases to form salt and water. A common example is aluminium. When aluminium is burnt or reacts with air, it forms aluminium oxide (Al₂O₃), which is an amphoteric oxide. Zinc also forms an amphoteric oxide (ZnO).
3. Which metals react very quickly with air, even without burning?
Metals that are high on the reactivity series, such as sodium (Na) and potassium (K), are extremely reactive. They react vigorously with the oxygen and moisture in the air even at room temperature. The reaction is so fast and exothermic (releases heat) that they can catch fire if left exposed. To prevent this, they are typically stored under kerosene oil.
4. Why don't metals like gold and silver tarnish or react when exposed to air?
Gold and silver are known as noble metals because they are very unreactive. They are located at the bottom of the reactivity series, which means they do not easily react with oxygen or other substances in the air under normal conditions. This chemical inertness is the reason they do not corrode or tarnish and are highly valued for making jewellery.
5. How is the process of burning a metal in air different from just leaving it exposed to air?
The key difference lies in the speed and conditions of the reaction.
- Exposing to air usually causes slow oxidation, or tarnishing, on the metal's surface over time. This forms a thin layer of metal oxide or carbonate, like the greenish coating on copper.
- Burning in air is a rapid, high-temperature combustion process. It is an intense chemical reaction that converts a significant amount of the metal into its oxide, releasing large amounts of energy as heat and light.
6. If aluminium is a reactive metal, why is it used for making aircraft parts and window frames that are constantly exposed to air?
Although aluminium is quite reactive, when it is first exposed to air, it instantly forms a very thin, tough, and non-porous layer of aluminium oxide (Al₂O₃) on its surface. This invisible protective layer is extremely stable and prevents the underlying aluminium metal from coming into contact with air and moisture, thus protecting it from further corrosion.
7. How can you demonstrate that the oxide formed by burning a metal like magnesium is basic in nature?
To demonstrate the basic nature of magnesium oxide, you can perform a simple test. First, collect the white ash (MgO) produced after burning a magnesium ribbon. Dissolve this ash in a small amount of water to form magnesium hydroxide (Mg(OH)₂), which is a base. Then, dip a strip of red litmus paper into the solution. The red litmus paper will turn blue, which is a definitive test confirming that the metal oxide is basic in nature.
8. How does the product of burning a metal in air differ from the product of burning a non-metal like carbon?
The primary difference is the chemical nature of the oxides formed.
- Metals (like magnesium or sodium) typically burn to form basic oxides. These oxides react with water to form bases (alkalis).
- Non-metals (like carbon or sulfur) burn to form acidic oxides. These oxides react with water to form acids, such as carbonic acid (from CO₂) or sulfurous acid (from SO₂).
























