




How Osmium’s Unique Characteristics Impact Science and Industry
The element Osmium in the periodic table is a shiny, silvery transition metal with atomic number 76 and Osmium atomic mass is 190.23 grams per mole in the periodic table. It belongs to Group 8, period 6, and block d of the periodic table. The electronic configuration of the element Osmium (Os) is given as $[Xe]$4f145d66s2.
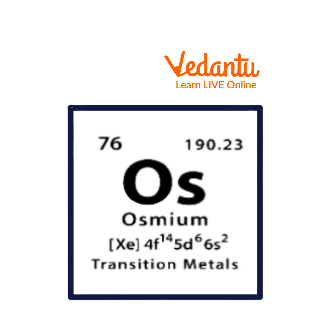
The Os Element
Osmium in the periodic table is the densest occurring element in nature. It occurs in the Platinum group and has the appearance of a bluish-white metal. With a mass fraction of barely 50 parts per trillion (ppt), the element osmium is one of the rarest elements in the crust of the Earth. The universe's estimated abundance of it is 0.6 parts per billion, making it the rarest precious metal.
History of the Osmium - Os Element
In 1803, Smithson Tennant mixed dilute aqua regia, a solution of nitric and hydrochloric acids, with platinum and noticed that not all of the metal dissolved. The residue was thought to be graphite by earlier experimenters, but he had a different suspicion and started looking into it. He eventually separated it into two new metal elements iridium and osmium by using a blend of acid and alkali treatments. He called the latter osmium because of the powerful odour it produced. Its name comes from the Greek word meaning smell, osme.
Although it was acknowledged as a novel metal, it wasn't utilised much since it was hard to work with and scarce. But it was used for gramophone needles and pen nibs for many years.
Physical Properties of Osmium
Osmium is a lustrous metal that is hard but brittle and is still lustrous at high temperatures. It is not very compressible.
Osmium atomic mass is 190.23 grams per mole.
The melting point of osmium is 3306 K (3033 °C, 5491 °F).
The boiling point of osmium is 5285 K (5012 °C, 9054 °F).
The Osmium density at room temperature is 22.59 grams per cubic centimeter.
Chemical Properties of Osmium
Oxidation states ranging from −2 to +8 are formed by osmium. The oxidation states +2, +3, +4, and +8 are the most prevalent.
The substance that exhibits the +8 oxidation state most frequently is osmium tetroxide. When osmium powder is exposed to air, this poisonous substance is created. It is a highly flammable, water-soluble, crystalline substance that is a light yellow colour and pungent. Osmium powder emits the distinctive aroma of osmium tetroxide.
In general, ligands that are good σ-donors (such as amines) and π -acceptors (heterocycles containing nitrogen) stabilise the lower oxidation states of osmium. Strong σ-donors and π-donors, such as oxide and nitride ions, stabilise the higher oxidation states.
Osmium resists attack by all acids, including aqua regia, in bulk form at ordinary temperatures and pressures despite having a wide variety of compounds in various oxidation states. However, fused alkalis attack osmium.
Seven isotopes of osmium are found naturally, five of which are stable: 187Os, 188Os, 189Os, 190Os, and (most prevalent) 192Os. With an alpha decay half-life of (2.0 ± 1.1) ✕ 1015 years or around 140000 times the age of the universe, 186Os can be regarded as stable in most situations. With a half-life of (1.12 ± 0.23) ✕ 1013 years, 184Os are also known to experience alpha decay. For all the other naturally occurring isotopes, alpha decay is expected, but this has never been seen, perhaps because of the extremely lengthy half-lives.
Below is a Table Representing the Oxidation States of Osmium.
Occurrence of Osmium
Osmium can be found in nature as an element or in natural alloys, particularly iridium-osmium alloys such as iridosmium and osmiridium (iridium rich) (osmium rich).
The platinum-group metals are found in nickel and copper deposits as sulphides, tellurides, antimonides, and arsenides; in each of these compounds, a negligible amount of iridium and osmium swap platinum.
Osmium can be discovered naturally in alloys with nickel or copper, just like all the other platinum-group metals.
The three types of geologic structures that have the highest concentrations of osmium within the Earth's crust are igneous deposits (crustal intrusions from below), impact craters, and deposits that were reworked from one of the former structures.
The Bushveld Igneous Complex in South Africa contains the largest known primary reserves.
Uses of Osmium
As a catalyst, Osmium is used in various industries.
Because of its high reflectivity, it is also extremely useful in the domain of space applications.
To produce more light, it is utilised in incandescent lamps and light bulbs.
To detect fingerprints, osmium tetroxide is utilised.
Since it can absorb hydrogen atoms, it is employed as an electrolyte in battery applications.
Fountain pen tips contain Osmium.
Precautions to be Taken while Handling Osmium
Osmium is a metal that is safe in its metallic state, but when it is finely divided, it becomes pyrophoric and forms volatile osmium tetroxide when it combines with oxygen at room temperature. Osmium tetroxide is extremely hazardous when in contact with the skin, inhaled, or consumed since it is also highly volatile and rapidly penetrates the epidermis. Osmium tetroxide should be handled under a fume hood since its low airborne concentrations can harm the skin or eyes and congest the lungs.
Osmium Applications
Osmium in its pure form is rarely used; rather this element is alloyed with other metal elements for high-wear uses like:
Alloys of osmium like osmiridium are tough and with some other metals within the group of platinum are utilised to make fountain pen tips, electrical contacts and instrument pivots. These elements were used to make phonograph styli tips during the early “45” and “LP” record era. The tips made out of osmium alloys are more long-lasting than chromium and steel, and were expensive than diamond and sapphire tips. Therefore, their use was stopped.
Osmium tetroxide is used in fingerprint detection and fatty tissue staining for electron and optical microscopy.
Oslamp was constructed with an osmium made filament by Auer von Welsbach in 1898.
Tetroxide and potassium osmate (the formers derivate) are essential oxidants in organic synthesis.
Similar to palladium, osmium in powder form absorb atoms of hydrogen and may help in making metal-hydride battery electrode.
Osmium has a clinical use called synovectomy for patients who have arthritis, in Scandinavia.
In 2011, two compounds osmium (II) and osmium (VI) were reported showing anticancer activities in vivo. Hence, it suggested a hopeful future to use compounds of osmium as anticancer medicines.
Osmium Price
Commonly, osmium metal is sold in the form of pure powder. Similar to other valuable metals, the unit of measure of osmium are grams and troy weight. As there is a minimal change in demand and supply of osmium, there is no alteration in the market price of osmium for decades. Moreover, since it is available in little amounts, working with this metal is challenging, has very fewer applications and it is difficult to keep it safe as it yields toxic compounds on oxidation.
The price of osmium per troy ounce has been the same from the 1990s. However, due to inflation, the metal has lost one-third of its price in the last two decades before 2019.
Safety Measures Related to Osmium
At room temperature, finely powdered osmium metal being pyrophoric, undergo reaction with oxygen and forms volatile osmium tetroxide. This produced compound can readily penetrate the skin and is harmful when inhaled, ingested or comes in contact with skin. Low concentrations of airborne osmium tetroxide vapour can lead to congestion in lung and skin, and damage the eyes. Hence, it must be used along with fume hoods.
Osmium tetroxide can be quickly reduced to nearly inert compounds using polyunsaturated vegetable oils or ascorbic acid.
Important Questions
What is the atomic number and electronic configuration of Osmium?
Ans: The atomic number of the element Osmium (Os) is 76 and the electronic configuration is $[Xe]$4f145d66s2.
Why is Osmium known as a d-block element?
Ans: The electronic configuration of Osmium is $[Xe]$4f145d66s2 and the last electron of Osmium enters the d subshell, so it is called a d-block element.
Name a few properties of the element Osmium.
Ans: Osmium is a lustrous metal that is hard but brittle. It has an atomic mass of 190.2 grams per mol, a melting point of 3045 °C, and a density of 22.59 grams per cubic centimetre.
Summary
In 1803 in England, Smithson Tennant made the first known discovery of Osmium. The element Osmium (Os) is a shiny, silvery transition metal with atomic number 76 and an atomic mass of 190.23 grams per mole. It belongs to Group 8, period 6, and block d of the periodic table. It is the densest element found in nature.
The electronic configuration of the element Osmium is given as $$[Xe]$$4f145d66s2. Osmium has -2 to +8 oxidation states and there are about seven isotopes of Osmium of which five are stable. The metal is unaffected by the action of acids and it is found to be used in the tips of a fountain pen.
Practice Questions
1. The element Osmium is affected by which of these
Water
Acid
None of the above
Answers: (b)
2. The element Osmium belongs to which period in the periodic table?
2
4
6
8
Answers: (c)
FAQs on Osmium: Definition, Properties & Applications
1. What is Osmium and where is it placed in the periodic table?
Osmium (symbol Os) is a chemical element with atomic number 76. It is a hard, brittle, bluish-white transition metal belonging to the platinum group. Located in group 8 and period 6 of the periodic table, it is known for being the densest naturally occurring element. Its properties make it one of the "noble metals" due to its resistance to corrosion and oxidation.
2. What are the key physical and chemical properties of Osmium?
Osmium has several distinct properties, making it a unique element. Key examples include:
- Density: It is the densest stable element, with a density of approximately 22.59 g/cm³.
- Appearance: It is a lustrous, bluish-white metal.
- Hardness: It is extremely hard and brittle, even at high temperatures.
- Melting/Boiling Points: It has one of the highest melting points (3033 °C) and boiling points (5012 °C) of all elements.
- Reactivity: In its solid form, it is highly resistant to acids and aqua regia. However, powdered osmium reacts with oxygen at room temperature to form the toxic and volatile Osmium Tetroxide (OsO₄).
- Oxidation States: It exhibits a wide range of oxidation states, from -2 to +8, which is the highest known for any element.
3. What is the electronic configuration of Osmium?
The electronic configuration of Osmium (Os), which has an atomic number of 76, is [Xe] 4f¹⁴ 5d⁶ 6s². This configuration places it in the d-block of the periodic table as a transition metal. The electrons in the 5d and 6s orbitals are its valence electrons, which participate in chemical bonding and give Osmium its characteristic properties.
4. What are the primary applications of Osmium?
Due to its extreme hardness, density, and corrosion resistance, Osmium is used in several highly specialised applications. The most common use is in alloys to create extremely hard and durable materials. Key examples of its applications include:
- Tips for fountain pens and ballpoint pens.
- Pivots for instrument and compass needles.
- Electrical contacts that require high durability.
- As a catalyst in various chemical reactions, such as ammonia synthesis.
- In forensic science, osmium tetroxide is used to stain fatty tissues for microscope analysis.
5. Where is Osmium found and how is it extracted?
Osmium is one of the rarest elements in the Earth's crust. It is not found as a pure element but occurs naturally in an alloy called osmiridium (an alloy of osmium and iridium). It is also found in platinum-bearing river sands and nickel-bearing ores. The commercial extraction of Osmium is a complex process that occurs as a by-product of nickel and copper refining.
6. Why is Osmium considered the densest element on Earth?
Osmium's remarkable density (22.59 g/cm³) is due to a combination of its high atomic mass (190.23 u) and the effects of the lanthanide contraction. The lanthanide contraction causes the atomic radius of elements following the lanthanide series to be smaller than expected. This means Osmium's heavy atoms are packed very tightly together in its crystal lattice structure, resulting in more mass per unit volume than any other element.
7. How does Osmium's hardness compare to a diamond, and what makes it so incompressible?
While diamond is known as the hardest naturally occurring material (measured by scratch resistance), Osmium is significantly more incompressible, meaning it resists being squeezed under immense pressure better than a diamond. Research has shown Osmium has a higher bulk modulus (a measure of resistance to compression) than diamond. This is because the metallic bonds in Osmium, involving its high density of valence electrons, are extremely strong and effectively resist compression.
8. Why is Osmium tetroxide (OsO₄) so toxic, and what precautions are necessary when handling the element?
While metallic osmium is relatively safe, its finely powdered form is pyrophoric and readily reacts with oxygen in the air to form Osmium tetroxide (OsO₄). This compound is highly toxic because it is extremely volatile and a powerful oxidizing agent. It can easily cross cell membranes and cause severe damage to the eyes, skin, and respiratory system, even at very low concentrations. Therefore, any work with powdered osmium or processes that might create OsO₄ must be done inside a fume hood with appropriate personal protective equipment (PPE).
9. What makes Osmium a "noble metal," and how does this affect its chemical reactivity?
Osmium is considered a noble metal, alongside elements like platinum, gold, and iridium, due to its exceptional resistance to corrosion and oxidation in its bulk form. This inertness is a result of its stable electron configuration and the high energy required to remove its valence electrons to participate in chemical reactions. This property means that solid Osmium does not react with common acids, including aqua regia, at room temperature, making it highly durable and non-reactive in most environments.
10. How do the unique properties of Osmium make it suitable for its specific, high-endurance applications?
Osmium's applications are a direct result of its unique combination of properties. Its extreme hardness and wear resistance are perfect for components that undergo constant friction, like the tips of fountain pens and instrument pivots. Its high melting point and corrosion resistance make it ideal for durable electrical contacts. Essentially, Osmium is chosen for applications where longevity, stability, and the ability to withstand physical stress are critical, and where other metals would quickly fail.























