




How Are Electrons Arranged in Atomic Shells?
The distribution of electrons in shells of the atomic orbitals of an atom is known as its electronic configuration. An atom consists of subatomic particles like protons, neutrons, and electrons. Protons and neutrons are present in the nucleus of an atom, while electrons are the ones that revolve around the nucleus of an atom in fixed orbits, which is explained by Bohr’s atomic model.
An atomic number of an element is equal to the number of electrons or protons (in a neutral atom, the number of electrons is equal to the number of protons) present in an atom. Now there’s a particular way these electrons are arranged in different orbitals, called the electronic configuration of an atom.
Bohr’s Atomic Model - Salient Features
The Bohr Atomic model states that a small, positively charged nucleus is surrounded by revolving, negatively charged electrons in set orbits. He concluded that an electron would have more energy if it is farther from the nucleus, whereas an electron would have less energy if it is closer to the nucleus. The postulates are as follows -
Electrons are negatively charged and revolve in a fixed circular path around the nucleus known as shells/orbits/energy level.
Each of these orbits/shells have a fixed energy level.
Integers such as n=1,2,3, and so on are used to represent the various energy levels. These are referred to as principle quantum numbers. The range of quantum numbers may change from the lowest energy level (nucleus side n=1) to the greatest energy level.
There are two methods to express the various energy levels or orbits, such as 1, 2, 3, 4..., and their corresponding shells/orbits are K, L, M, and N... shells. The term "ground state" refers to the electron's lowest energy level.
When an electron in an atom needs to go from a lower to a higher energy level, it gains the necessary energy, and when it needs to move from a higher to a lower energy level, it loses energy.
How are Electrons Distributed in Different Orbits/Shells?
As we know, outside of an atom's nucleus, a cloud of negatively charged subatomic particles called electrons are distributed in different shells. The potential energy in various orbits determines their arrangement. The different energy levels are referred to as 1, 2, 3, 4… and the equivalent shells are referred to as K, L, M, N, and so on.
Starting in the centre, the shells progressively spread outward. K-shell will have the least energy. The L shell will have more energy than the K shell since it is somewhat further from the nucleus. The energy level will be highest in the outermost shell. Any atom will be most stable when it has the least energy.
To reach the state of least energy, an atom must first occupy the lowest energy level. The higher energy levels will eventually fill with electrons. As a result, electrons will begin to fill the K shell before moving on to the L, M, N, and so on.
Bohr-Bury Scheme
The Bohr-Bury Scheme determines how the electrons are distributed throughout the orbits or shells of the atoms. The concept behind the Bohr-Bury Scheme is as follows:
The electrons in an atom are grouped in several shells around the nucleus, and they first inhabit the shell closest to the nucleus since it has the lowest energy.
The first and innermost shell is known as the K-shell, which may accommodate up to two electrons.
The L-shell, the second shell, may hold up to eight electrons.
A total of eighteen electrons can fit in the third shell, known as the M-shell.
The total or the maximum number of electrons in each shell can be given by the formula 2n2, where n (n=1,2,3,4….) is the shell number or the principal quantum number.
Filling Up of Atomic Orbitals
It is governed by 3 principles, namely:
Aufbau Principle: According to it, electrons occupy atomic orbitals in ascending order of orbital energy level, meaning that lower energy levels are occupied before higher energy levels.
Pauli’s Exclusion Principle: Pauli's Exclusion Principle states that no two electrons in the same atom may have the same values for all four of their quantum numbers.
Hund’s Rule: Before becoming doubly occupied, every orbital in a subshell is solely filled by one electron, and all of the electrons in singly occupied orbitals have the same spin.
Examples
1. Helium
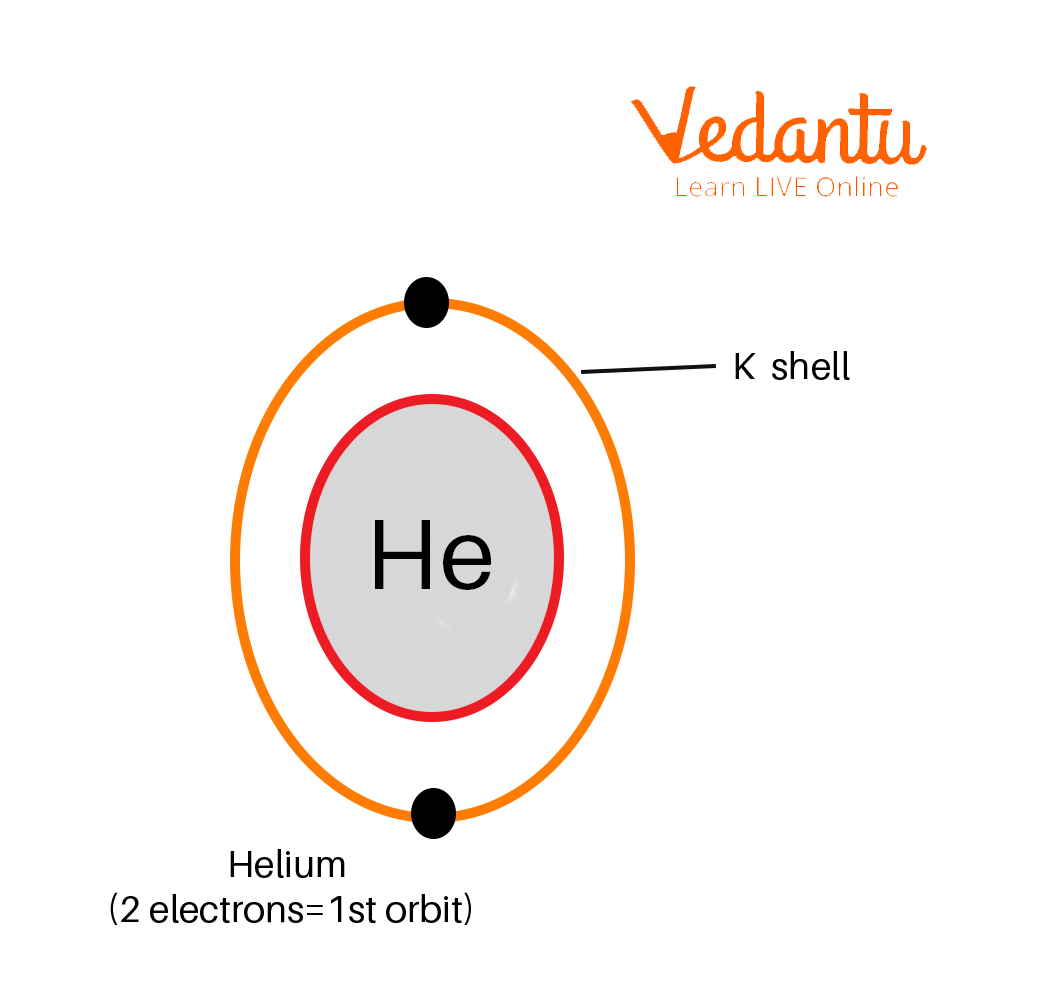
Orbital diagram of Helium
Atomic number = 2
Number of electrons = 2
Electronic configuration = 2
The K shell can occupy a maximum of 2 electrons hence only 1 Shell, which is the K shell, is required to distribute the 2 electrons of Helium.
2. Oxygen

Orbital Diagram of Oxygen
Atomic number = 8
Number of electrons = 8
Electronic configuration = 2,6
K shell (1st orbit) may hold a maximum of 2 electrons. The remaining electrons (6 electrons) will fit into the second orbit, which is the L shell and can accommodate a maximum of 8 electrons. Hence, Oxygen requires a total of 2 shells to distribute its electrons.
Important Questions
1. What is electronic configuration?
Ans: The arrangement or distribution of electrons in the shells of an atom is defined as the electronic configuration of an element.
2. What are shells/orbits in an atom?
Ans: Electrons revolve around the nucleus in a fixed circular path known as shells/orbits. There are K, L, M, N…..shells, and these can also be represented as integers based on the principal quantum number.
Summary
Subatomic particles called electrons are negatively charged and distributed outside the atom's nucleus in fixed orbits/shells. Based on their potential energies in various orbits, the arrangement is determined. Electronic configuration describes how the electrons are distributed throughout the energy shells.
It is based on the Bohr-Bury scheme, which states that the maximum number of electrons that may be found in a specific energy shell of an atom is determined by the formula 2n2, where n is the number of energy shells. 1, 2, 3, and 4 are the numbers that represent the various energy levels. The designations for the corresponding shells are K, L, M, N, and so on.
Practice Questions
1. How many shells are required to distribute the 17 electrons found in chlorine atoms?
1
2
3
4
2. Which of the following principles govern the filling up of the atomic orbitals?
Aufbau principle
Pauli’s exclusion principle
Hund’s rule
All of the above
Answers:
(c)
(d)
FAQs on Electron Distribution in Different Orbits and Shells
1. What is electronic configuration and how is it determined for an element?
Electronic configuration refers to the specific arrangement or distribution of electrons in the different energy shells or orbits of an atom. To determine it, you first find the total number of electrons (which equals the atomic number in a neutral atom). Then, you distribute these electrons into shells starting from the one closest to the nucleus (K-shell), following specific rules like the Bohr-Bury scheme, ensuring that lower energy shells are filled first.
2. What formula is used to calculate the maximum number of electrons a shell can hold?
The maximum number of electrons that can be accommodated in a particular shell is given by the formula 2n², where 'n' is the shell number or the principal quantum number. For example:
- For the first shell (K-shell, n=1), the maximum number of electrons is 2(1)² = 2.
- For the second shell (L-shell, n=2), the maximum number of electrons is 2(2)² = 8.
- For the third shell (M-shell, n=3), the maximum number of electrons is 2(3)² = 18.
3. What are the key rules of the Bohr-Bury scheme for distributing electrons?
The Bohr-Bury scheme provides a set of rules for filling electrons in different orbits. The main rules are:
- The maximum number of electrons in any shell is determined by the formula 2n².
- Electrons always fill the lowest energy levels first. This means the K-shell (n=1) is filled before the L-shell (n=2), and so on.
- The outermost shell of an atom cannot accommodate more than 8 electrons, even if it has the capacity to hold more. This is known as the octet rule and is key to an atom's stability.
4. Why do electrons fill the K-shell first before moving to outer shells like the L-shell?
Electrons fill the K-shell first because it is the lowest energy level in an atom. All systems in nature tend to seek a state of maximum stability, which corresponds to the minimum possible energy. The K-shell is closest to the positively charged nucleus, making it the most stable shell. Therefore, electrons occupy this lowest energy shell completely before any electrons begin to fill the next higher energy level, the L-shell.
5. How does an element's electronic configuration explain its chemical reactivity?
Electronic configuration is fundamental to understanding chemical reactivity because it determines the number of valence electrons an atom has. These are the electrons in the outermost shell (valence shell). An atom's desire to achieve a stable state (usually a full valence shell with 8 electrons) drives its chemical behaviour. Atoms with incomplete valence shells will tend to gain, lose, or share electrons to achieve this stability, which is the basis for forming chemical bonds and participating in reactions.
6. What is the difference between an atom's orbit and an orbital?
The terms are often confused but describe different concepts. An orbit, as proposed by the Bohr model, is a well-defined, two-dimensional circular path around the nucleus where an electron revolves. In contrast, an orbital, based on the modern quantum mechanical model, is a three-dimensional region of space around the nucleus where the probability of finding an electron is highest. Orbits suggest a fixed path, while orbitals define a probable location.
7. What are the main limitations of using the Bohr model to explain electron distribution?
While useful for simple atoms like hydrogen, the Bohr model has several key limitations:
- It violates Heisenberg's Uncertainty Principle by defining both the exact position and momentum of an electron simultaneously.
- It fails to accurately predict the electron spectra for larger, multi-electron atoms.
- It cannot explain the splitting of spectral lines in the presence of a magnetic field (Zeeman effect) or an electric field (Stark effect).





































