




Introduction to Tollens’ and Fehling’s Test
As we already know, since aldehydes and ketones both possess the carbonyl functional group, they undergo similar chemical reactions. But aldehydes differ from ketones in their oxidation reactions. Thus, oxidation is one of the methods to distinguish aldehydes from ketones. Aldehydes are easily oxidised to carboxylic acids on treatment with common oxidising agents like nitric acid, potassium permanganate, potassium dichromate, etc. and even mild oxidising agents also oxidise aldehydes.
$R-CHO\xrightarrow{[O]}R-COOH$
Ketones are oxidised generally under vigorous conditions, i.e., strong oxidising agents and at elevated temperatures. Their oxidation involves carbon-carbon bond cleavage to afford a mixture of carboxylic acids having a lesser number of carbon atoms than the parent ketone.
In this article, we are going to learn about the most common mild oxidising agents, i.e., Tollens’ reagent and Fehling’s reagent which are used as Tollens’ test and Fehling’s test to distinguish aldehydes from ketones.
Note: 👉Prepare for Your Future in Medicine with the NEET Rank and College Predictor 2025.
What is Tollens’ Test?
Tollens’ test is one of the tests used to distinguish between aldehydes and ketones. This test is mostly given by the compounds containing the aldehyde group. As a silver mirror is produced at the end of the reaction due to the formation of silver metal, it is also called a silver mirror test in laboratories. The reagent which is used in this test is known as Tollens’ reagent. Tollens’ reagent is a solution of freshly prepared ammoniacal silver nitrate.
Tollens’ Reagent
The Tollens’ reagent is a solution of freshly prepared ammoniacal silver nitrate with the chemical formula Ag(NH3)2OH. It consists of silver nitrate, ammonia, and sodium hydroxide. Tollens’ reagent is named after his discoverer, Bernhard Tollens’. It is freshly prepared in the laboratory directly as it has a short shelf life and cannot be sold commercially.
Procedure of Tollens’ Test
The Tollens’ test is done by first preparing the Tollens’ reagent, i.e., ammoniacal silver nitrate Ag(NH3)2OH and then adding the respective sample to it.
Step-1 To prepare the Tollens’ reagent, add aqueous silver nitrate to aqueous sodium hydroxide.
$AgN{{O}_{3}}+NaOH\to AgOH+NaH{{O}_{3}}$
$2AgOH\to A{{g}_{2}}O+{{H}_{2}}O$
Step-2 Now, add aqueous ammonia dropwise until the precipitated silver oxide gets completely dissolved.
\[A{{g}_{2}}O+4N{{H}_{3}}+{{H}_{2}}O\to 2Ag(N{{H}_{3}})_{2}^{+}+2O{{H}^{-}}\]
Thus, Tollens’ reagent is prepared. Now, add this freshly prepared Tollens’ reagent to the respective sample solution.
$R-CHO+2{{\left[ Ag{{\left( N{{H}_{3}} \right)}_{2}} \right]}^{+}}+3\overline{O}H\to R-CO\overline{O}+2Ag+2{{H}_{2}}O+4N{{H}_{3}}$
On treatment with Tollens’ reagent, aldehydes get oxidised to corresponding carboxylic acids and a bright silver mirror is produced due to the formation of silver metal. Ketones are not oxidised by Tollens’ reagent. Thus, Tollens’ test is used to distinguish between aldehydes and ketones.
What is Fehling’s Test?
A Fehling's test is one of the tests used to distinguish aldehydes from ketones and aliphatic aldehydes from aromatic aldehydes. In Fehling’s test, a reddish-brown precipitate is obtained. The reagent which is used in this test is known as Fehling’s reagent. Fehling’s reagent is a mixture of two different solutions known as Fehling’s A and Fehling’s B.
Fehling’s Reagents
Fehling’s reagent is basically composed of two solutions which are Fehling’s A and Fehling’s B. Fehling’s A is a solution of aqueous copper sulphate and Fehling's B is a solution of alkaline sodium potassium tartrate which is commonly known as Rochelle salt. These two solutions are mixed in equal amounts before the test and together are called Fehling’s reagents.
Procedure of Fehling’s Test
The Fehling’s test is done by first preparing the Fehling’s reagent which is a mixture of Fehling’s A and Fehling’s B and then adding the respective sample to it.
To prepare Fehling’s reagent, mix Fehling’s A and Fehling’s B in equal amounts with a strong alkali, usually sodium hydroxide.
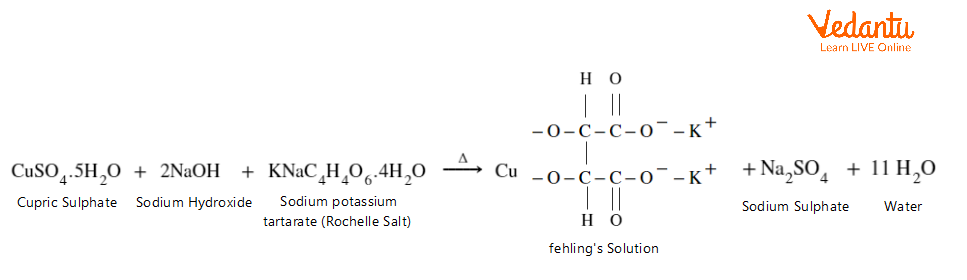
Thus, Fehling’s reagent is prepared. Now, add this freshly prepared Fehling’s reagent to the respective sample solution.
$R-CHO\text{ }+\text{ }2C{{u}^{2+}}\text{ }+\text{ }5\overline{O}H\to R-CO{{O}^{-}}\text{ }+\text{ }\underset{\text{Red brown ppt}}{\mathop{C{{u}_{2}}O}}\,\text{ }+\text{ }3{{H}_{2}}O$
On treatment with Fehling’s reagent, aldehydes get oxidised to corresponding carboxylate anions with a red-brown ppt. Aromatic aldehydes and ketones do not respond to this test. Thus, Fehling’s test is used to distinguish aldehydes from ketones and aliphatic aldehydes from aromatic aldehydes.
Summary
Tollens’ and Fehling’s tests are used to distinguish between aldehydes and ketones. In Tollens’ test, Tollen’s reagent which is a solution of ammoniacal silver nitrate is added to the respective sample. If the given sample contains an aldehyde group, then it gets oxidised to corresponding carboxylic acids and a bright silver mirror is produced due to the formation of silver metal. Whereas if the given sample contains a ketone group, then there is no response to the test.
In Fehling’s test, Fehling’s reagent is added to the respective sample. Fehling’s reagent is the mixture of two solutions in which Fehling’s A is aqueous copper sulphate and Fehling’s B is alkaline sodium potassium tartrate. If the given sample contains an aldehyde group, then it gets oxidised to the corresponding carboxylate anion and a red-brown precipitate is formed. Whereas, if the given sample contains a ketone group or aromatic aldehyde group, then there is no response to the test.
FAQs on Tollens’ Test and Fehling’s Test - NEET Important Topic
1. What is Tollens’ test? Which solution is used as a reagent in Tollen’s test? Also, explain the result of this test.
Tollens’ test is used to distinguish aldehydes from ketones. In this test, an ammoniacal silver nitrate solution is used as a reagent which is called Tollens’ reagent. When a Tollens’ reagent is added to the sample containing the aldehyde group, it gets oxidised to the corresponding carboxylic acid and a bright silver mirror is produced. Whereas, when it is added to a sample containing the ketone group, there is no response to the test. Thus, in this way, aldehydes can be differentiated from ketones using the Tollen’s test.
2. What is Fehling’s test? Which solution is used as a reagent in Fehling’s test? Also, explain the result of this test.
Fehling’s test is used to distinguish aldehydes from ketones and aliphatic aldehydes from aromatic aldehydes. In this test, a solution mixture of aqueous copper sulphate and alkaline sodium potassium tartrate is used as a reagent which is known as Fehling’s reagent. When a Fehling’s reagent is added to the sample containing the aldehyde group, it gets oxidised to the corresponding carboxylate anion and a red-brown precipitate is formed. Whereas, when it is added to a sample containing the ketone group or aromatic aldehyde group, there is no response to the test.
























