




Introduction to Coordination Compounds
Chemical compounds made up of complex anion and simple cation or complex cation and simple anion, or complex anion and complex cation are known as coordination compounds. The central metal in the complex ions is connected to the ligands via coordinate covalent bonds formed by the electron pairs donated by the ligands. Chlorophyll, hemoglobin, vitamin B12 are some of the naturally occurring coordination compounds. Hence, we can say life would have been impossible without coordination compounds.
Coordination compounds are the colorful stable compounds that show specific magnetic properties, due to which they are widely used in biochemistry, extraction of metals, dyes and pigments industry, medines, as catalysts etc.
Important Topics of Co-ordination Compounds:
Double salts and Coordination compounds
IUPAC nomenclature of coordination compounds
Werner’s Theory
Valence Bond Theory (VBT)
Crystal Field Theory (CFT)
Magnetic Properties of coordination compounds
Isomerism in coordination compounds
Ambident ligand
Homoleptic and heteroleptic complex
chelating effect
Denticity
Important Definitions of Coordination Compounds
Some Important Terms Pertaining to Coordination Compounds:
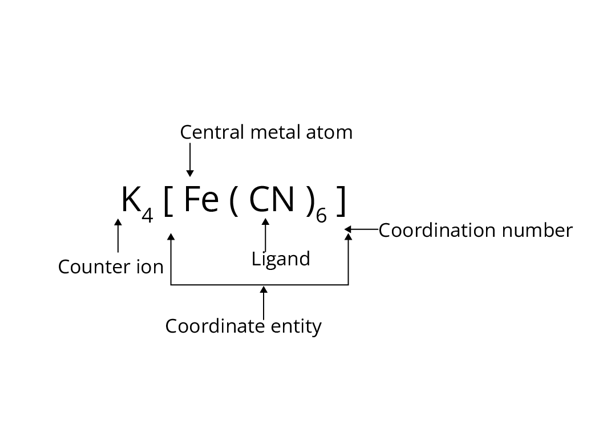
1. Coordination Entity:
Coordination entity constitutes the central metal ion bonded to a fixed number of molecules or ions.
2. Central Atom or Ion:
In coordination sphere, the fixed number of molecules or ions are bound to the atom or
ion in a definite geometrical arrangement. In complex K4[Fe(CN)6], the central ion is Fe2+.
3. Ligand:
Ions or molecules which are bound to a central metal or ion by coordinate bond which is formed by donated electron pairs by the atom of a molecule or ion known as ligand.
Unidentate Ligand:
In coordination chemistry, the donor atom of a ligand of coordinate compounds is bound to central metal through the single donor atom, the ligand is known as unidentate. For example- Cl–, H2O, NH3.
Bidentate Ligand:
In coordination chemistry, the donor atom of a ligand of coordinate compounds is bound to central metal through the two donor atoms, the ligand is known as bidentate. For example - ethane-1,2-diamine (H2NCH2CH2NH2), oxalate ion (C2O4–2-).
Polydentate Ligand:
When the ligand ion or molecule is bound to central metal through the two or more than two donor atoms, for example -EDTA–4.
EDTA–4: It is a hexadentate ligand, has 6 atoms of ligand molecule to form bonds with central metal. The O atom of 4 carboxylic group and 2 N atoms of amine group acts as donon atom as shown below:
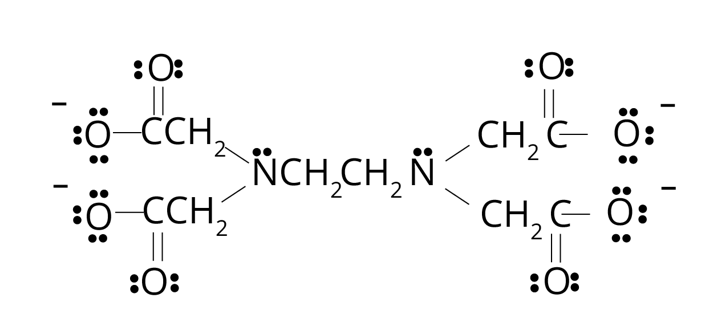
EDTA is a chelating agent which forms four or six bonds with the central metal ion to show chelates.
4. Coordination Number:
Coordination number of the complex ion is the number of donor atoms of the ligand bonded to the central metal ion. In complex K4[Fe(CN)6], 6 atoms of cyanide ligand are attached to Fe2+ ion hence, the coordination number for the complex is 6.
Isomerism in Coordination Compounds:
Isomers are the compounds which have the same molecular formula but different chemical formula. Let's discuss the isomerism of coordination compounds with examples.
1. Structural Isomerism:
Ionisation Isomerism:
Ionisation isomerism occurs when the compounds have different counter ions to produce different ions in the solution.
The examples of coordination compounds showing ionisation isomerism: [Co(NH3)5(Br)]SO4 and [Co(NH3)5(SO4)]Br are ionisation isomers that have SO4 2– and Br– counter ions.
Linkage Isomerism:
This isomerism occurs in the compound containing ambident ligands such as SCN, CN–, NO2– as they have two donor atoms and can be bonded to metal form either of atoms of ligand.
The examples of coordination compounds showing linkage isomerism: [Co(NH3)5(NO2)]Cl2 and [Co(NH3)5(ONO)]Cl2. Ligand is linked through N or O atoms in the isomers.
Coordination Isomerism
The isomerism occurs in complex cation and complex anion in which the central metal ions of cation and anion exchanges.
The examples of coordination compounds showing coordination isomerism: [Co(NH3)6][Cr(CN)6] and [Cr(NH3)6][Co(CN)6]
Hydrate or Solvate Isomerism:
This isomerism occurs in the compound containing water molecule . The water molecule in the isomers can be present as ligand or present as free molecule.
The examples of coordination compounds showing hydrate or solvate isomerism: [Cr(H2O)6]Cl3] and [Cr(H2O)5Cl]Cl2,H2O
2. Stereoisomerism:
Geometrical Isomerism:
This isomerism occurs in the compounds which have 4 and 6 coordination number.
(MA2B2) type of complex is a square planar complex which exhibits geometrical isomerism where A and B are unidentate ligands.
![[MA2B2] type of complex is a square planar complex which exhibits geometrical isomerism where A and B are unidentate ligands.](https://vedantu.com/seo/content-images/e4ab5be2-fe76-4cca-b20d-9f8d7342e291.png)
(MA2B4) type complex is an octahedral complex which exhibits geometrical isomerism where A and B are unidentate ligands.
![[MA2B4] type complex is an octahedral complex which exhibits geometrical isomerism where A and B are unidentate ligands.](https://vedantu.com/seo/content-images/9fed0785-c307-476d-b1d4-b801488143dc.png)
((AA)2B2) type complex is an octahedral complex which exhibits geometrical isomerism where AA is bidentate ligand and B is unidentate ligand.
![[M(AA)2B2] type complex is an octahedral complex which exhibits geometrical isomerism where AA is bidentate ligand and B is unidentate ligand.](https://vedantu.com/seo/content-images/ad0bc205-6fd7-4c49-a5db-9bb27c1e8291.png)
(MA3B3) type complex is an octahedral complex which exhibits geometrical isomerism where A and B are unidentate ligands.
![[MA3B3] type complex is an octahedral complex which exhibits geometrical isomerism where A and B are unidentate ligands.](https://vedantu.com/seo/content-images/359975eb-2765-4102-ac2c-6bf866edc2b3.png)
Optical Isomerism:
This isomerism occurs due to non-superimposable mirror images formed due to chirality and no plane of symmetry in the compound. Chirality in the molecule generally occurs due to attachment of different groups to the carbon atom. The plane of symmetry is the imaginary line that bisects the molecule. The two forms of isomerism are dextro(d) and leavo(l).
(M(AA)3) type complex is an octahedral complex which exhibits optical isomerism where AA is bidentate ligand due to no plane of symmetry.
![[M(AA)3] type complex is an octahedral complex which exhibits optical isomerism where AA is bidentate ligand due to no plane of symmetry.](https://vedantu.com/seo/content-images/3da416ea-2446-4e2d-bcff-38c5672f6bd7.png)
(M(AA)2B2) type complex is an octahedral complex which exhibits optical isomerism where AA is a bidentate ligand and B is a unidentate ligand.
![[M(AA)2B2] type complex is an octahedral complex which exhibits optical isomerism where AA is a bidentate ligand and B is a unidentate ligand.](https://vedantu.com/seo/content-images/31c49a3b-4324-4e8e-b711-24413f2d0d6c.png)
Valence Bond Theory(VBT):
This theory explained the orbital hybridisation in the complex as the penultimate d orbital close to s and p orbital hybridized to form the complex when the ligand approaches the central metal ion. This theory helps to predict the geometry of the complex.
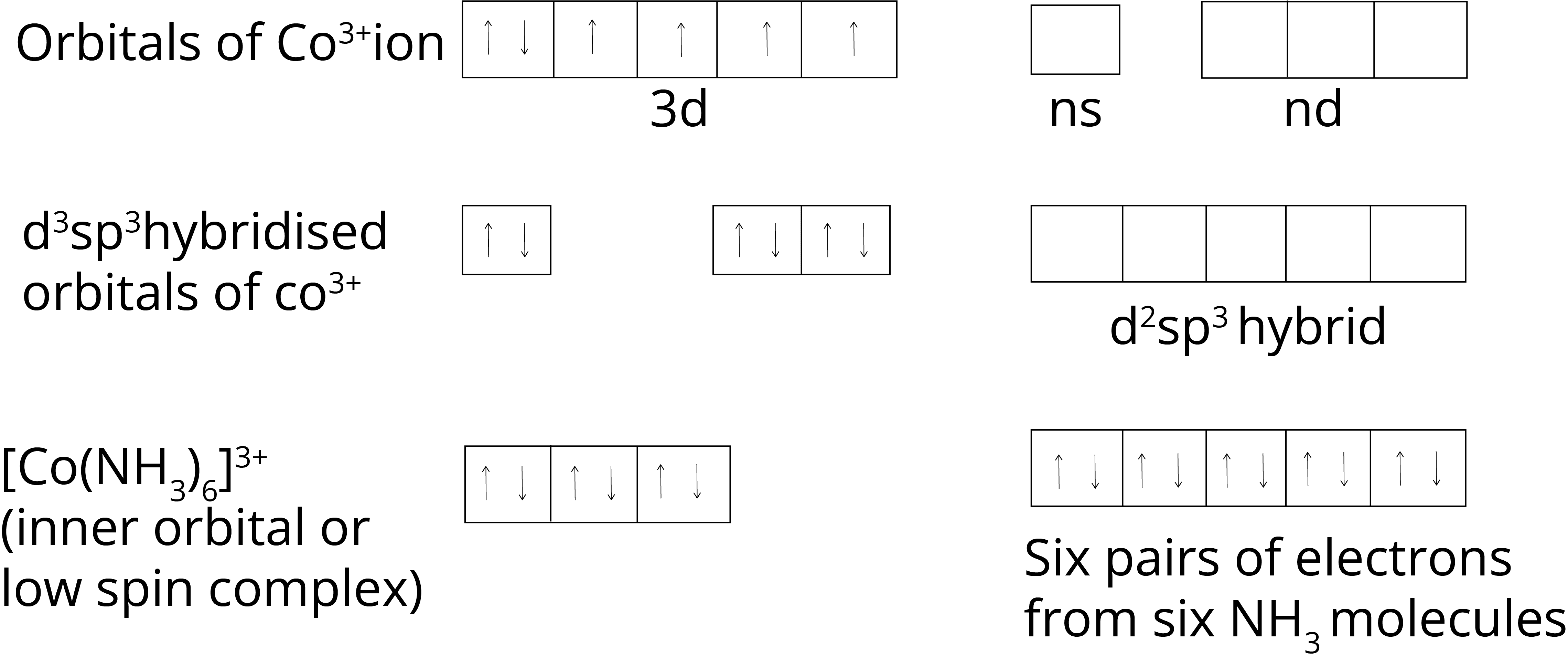
Inner Orbital or Low Spin Complex: When the inner d orbitals are used in hybridisation of the complex inner orbital or low spin complex are formed .
In complex [Co(NH3)6]3+, Co metal has +3 oxidation state, so the electronic configuration is 3d6, when the NH3 ligand approaches the central metal ion, inner 3d orbital hybridisation to form the complex. As all the electrons are paired in the complex, it is a diamagnetic complex.
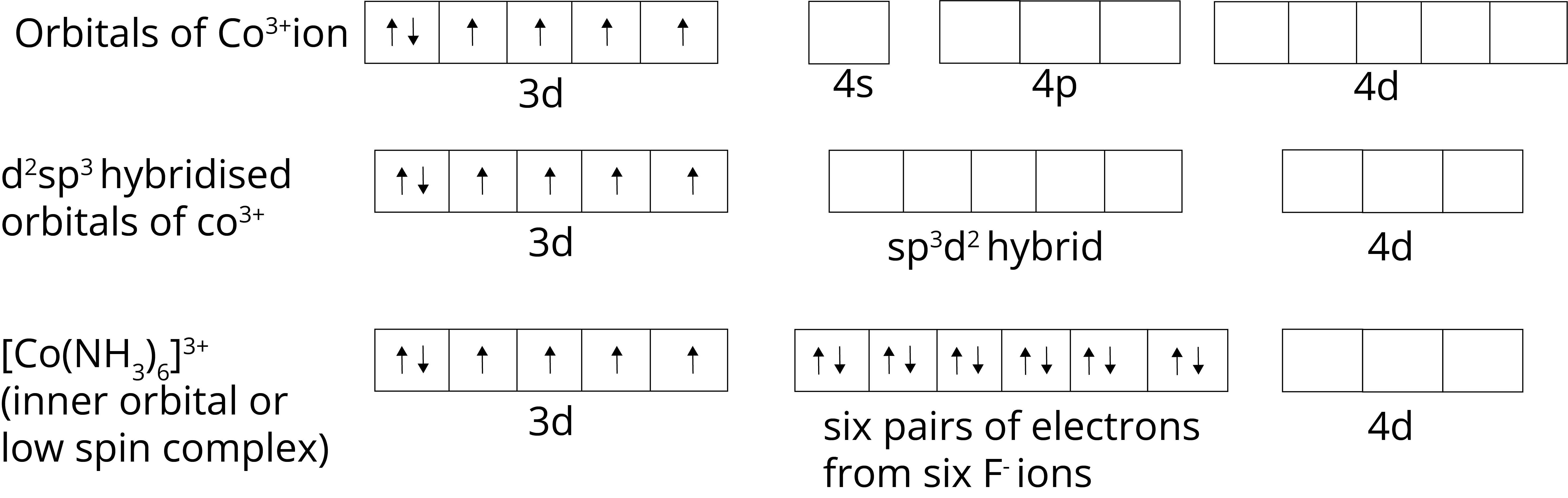
Outer Orbital Complex or High Spin Complex: When the outer d orbitals are used in hybridisation of the complex outer orbital or high spin complex are formed. In complex [CoF6]3–, Co metal has +3 oxidation state, so the electronic configuration is 3d6, when the F– ligand approaches the central metal ion, outer 3d orbital hybridisation to form complex. As the complex has unpaired electrons, it is a paramagnetic in nature.
Magnetic Properties of Coordination Compounds:
The coordinate compound can be characterized as diamagnetic and paramagnetic compounds.
Diamagnetic compounds have all the electrons paired in the complex.
Paramagnetic compounds have at least one unpaired electron in the complex.
Spin only magnetic moment is given by $\sqrt{n(n+1)}$, where n is the number of unpaired electrons.
Postulates of VBT:
Theory could not explain the inner orbital and outer orbital complex on the basis of strong and weak ligands.
It could not explain the color exhibited by the complex.
It has many assumptions.
It could not distinguish between the tetrahedral and square planar geometry existed by the complex.
It could not explain the thermal or kinetic stability of the complex.
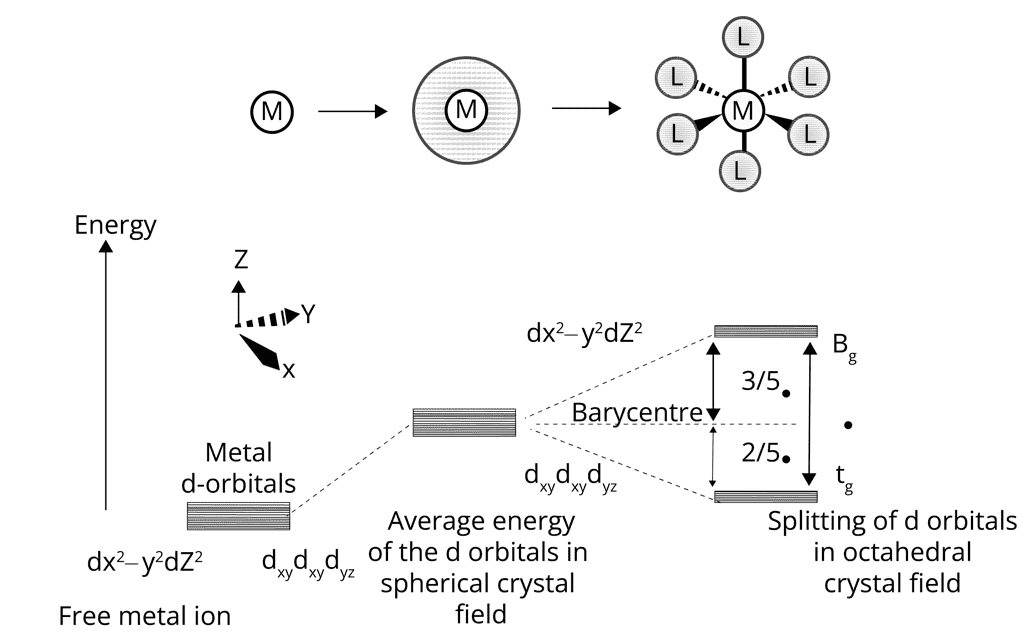
Crystal Field Theory (CFT):
Theory is based on the electrostatic bonding model between metal- ligands.
According to CFT, the d orbital in the complex in the presence of ligands degenerate.
In octahedral crystal field, d orbital degenerate to yield three orbitals of lower energy, t2g and two orbitals of higher energy, eg.
In the tetrahedral crystal field, d orbital degenerates to yield two orbitals of lower energy, eg and three orbital of higher energy, t2g.
(image will be uploaded soon)
Spectrochemical Series:
(Weak field ligand) I– < Br– < Cl– < NO2– < F– < OH– < C2O42–< H2O < EDTA4– < NH3 < ethylenediamine (en) <NO2 < CN– < CO (Strong field ligand).
Effective Atomic Number (EAN):
EAN is the total number of electrons present in the central atom along with the electrons gained from the ligands. It represents the electronic configuration of the nearest noble gas.
EAN = Z - O.N. + 2 (C.N.)
Z : Atomic number of of the central atom
O : Oxidation number of the central atom
C.N. : Coordination number of the central atom
Solved Examples From the Chapter
Example 1: Co-ordination number of Co in [Co(NH3)6]2+ is:
(a) 4
(b) 5
(c) 6
(d) 8
Solution (b) The compound, [Co(NH3)6]2+ which is a coordination compound has 6 NH3 ligands attached to the Cobalt (Co) metal , therefore, the coordination number of Co is 6.
Trick: Co-ordination number is equal to total number of ligands in a complex.
Example 2: Which of the following complexes will show geometrical as well as optical isomerism? en = ethylenediamine
(A) [Pt(NH3)2Cl2]
(B) [Pt(NH3)Cl4]
(C) [Pt(en)3}4+
(D) [Pt(en)2Cl2]
Solution: en is a bidentate ligand and Cl is a unidentate ligand. [Pt(en)2Cl2] which is a coordination compound of type [M(AA)2B2] complex that will show geometrical as well as optical isomerism.
Solved Questions from the Previous Year Question Papers
Question 1: The geometry and magnetic behavior of the complex [Ni(CO)4]
(a) Square planar geometry and paramagnetic
(b) Tetrahedral geometry and diamagnetic
(c) Square planar geometry and diamagnetic
(d) Tetrahedral geometry and paramagnetic
Solution: (b) In [Ni(CO)4], which is a coordination compound, the Ni is in zero oxidation state and CO is a strong field ligand, so it forms inner orbital complex as shown below:
(image will be uploaded soon)
Hybridisation of [Ni(CO)4] is sp3 and hence, it has tetrahedral geometry and all the electrons are paired hence, it is a diamagnetic complex.
Question 2: The type of isomerism shown by the complex [CoCl2(en)2] is-
(a) Ionization isomerism
(b) Coordination isomerism
(c) Geometrical isomerism
(d) Linkage isomerism
Solution: The type of isomerism shown by the complex [CoCl2(en)2] is geometrical isomerism as shown below:
![The type of isomerism shown by the complex [CoCl2(en)2] is geometrical isomerism](https://vedantu.com/seo/content-images/08e6aca0-dc3a-4f51-91da-7ca0f48735f4.png)
Question 3: Ethylene diaminetetraacetate (EDTA) ion is :
Tridentate ligand with three "N" donor atoms
hexadentate ligand with four "O" and two "N" donor atoms
Unidentate ligand
Bidentate ligand with two "N" donor atoms
Solution:(b) EDTA ion is a hexadentate ligand, has 6 atoms of ligand molecule to form bonds with central metal. The O atom of 4 carboxylic group and 2 N atoms of amine group acts as donon atom
Practice Questions
Question 1: The complex [Co(NH3)6][Cr(CN)6] and [Cr(NH3)6] [Co(CN)6] are the examples of which type of isomerism ?
(a) Linkage isomerism
(b) Ionization isomerism
(c) Coordination isomersim
(d) Geometrical isomerism
Answer: (a) Coordination isomerism
Question 2: Considering H2O as a weak field ligand, the number of unpaired electrons in [Mn(H2O)62+ will be (At. no. of Mn = 25) :-
(a) Four
(b) Three
(c) Five
(d) Two
Answer: (c) Five
Conclusion:
In coordination compounds, the central atom or ion is connected to the ligands, and the ligands give electron pairs to create coordinate covalent bonds.. Coordinate compounds on the basis of the number and type of ligand and counter ions attached to the central atom or ion shows isomerism. There were many theories to explain the structure, shape, geometry, magnetic property, color and stability of compounds.
In the NEET competitive exam, coordination compound is one of the important topics of inorganic chemistry, which is of moderate to easy level in terms of difficulty level.
NEET Chapter- Coordination Compounds

 Share
ShareFAQs on NEET Chapter- Coordination Compounds
1. What is coordination compound with example?
Coordination compounds are the compounds that contain a central atom or ion attached to ligand molecule or ion by coordinate covalent bond, formed by electron pair donation by the ligand. Coordinate compounds give complex ions in solution.
Example: [Pt(NH3)2Cl2], K3[Fe(CN)6] and [Fe(CO)5]
2. What are the important topics in coordination compounds?
Important topics of coordination compounds are:
Coordination number
Oxidation
Magnetic moment and magnetic properties of compound
Color of compound
Homoleptic and heteroleptic compound
Types of ligands
Nomenclature of coordination compounds
Isomerism in coordination compounds
3. Is coordination compounds important for NEET?
Yes, coordination chemistry is one of the important topics of chemistry, also for NEET exam. 3 questions of coordination compounds are asked in the paper, which in total is of 12 marks, so it is an important topic for NEET exam.




















 Watch Video
Watch Video


