




NEET Notes on Amines - Important Concepts and Properties
Amines can be found in proteins, vitamins, alkaloids, and hormones in nature. Polymers, dyestuffs, and pharmaceuticals are examples of synthetic materials. To raise blood pressure, two biologically active chemicals, adrenaline and ephedrine, both of which contain a secondary amino group, are utilized. In dentistry, novocain, a synthetic amino molecule, is used as an anesthetic.
In this article, we have covered all of the main aspects of amine, amide, and nitro compounds. Students can refer to this amine neet notes and clear their basic knowledge on this chapter.
Important Topics of Amines
Aliphatic amine
Carbylamine reaction
Coupling reaction
Amide
Hoffman bromamide reaction
Aromatic amine
Nitro camide compounds
Nitration
Diazonium salt
Important Concepts of Amines
What are Amines?
Amines are ammonia derivatives that are made by replacing one, two, or all three hydrogen atoms with alkyl and/or aryl groups.
Structure of Amine
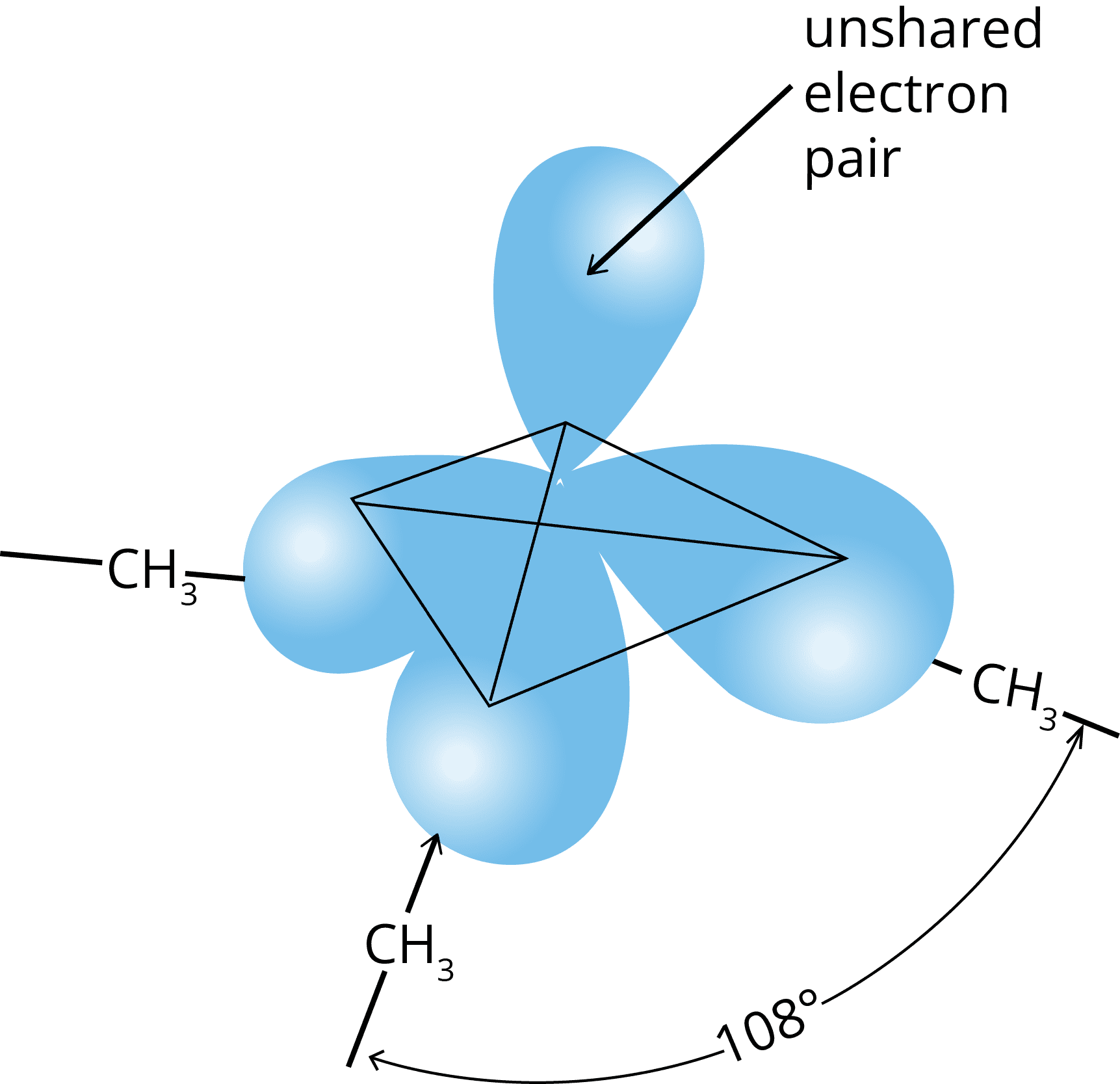
Pyramidal Shape of Trimethylamine
Types of Amine
Depending on the quantity of hydrogen atoms in ammonia molecules that are replaced by alkyl or aryl groups. RNH2 or ArNH2, a primary amine, is formed when one hydrogen atom of ammonia is replaced by R or Ar (1o).
If two hydrogen atoms of ammonia or one hydrogen atom of R-NH2 are substituted by another alkyl/aryl(R') group, secondary (2o) amines (R2NH) are formed.
Tertiary (3o) Amines- Tertiary amines are formed when another hydrogen atom of secondary amine is replaced by an alkyl/aryl group (R3N).
Properties of Organic Compounds Containing Nitrogen (Amines)
Lower (amines with less carbon) aliphatic amines exist in gaseous state with a fishy odor.
Aniline and other arylamines are generally colourless, but due to air oxidation, they become coloured with storage.
Because they can establish hydrogen bonds with water molecules, lower aliphatic amines are soluble in water. However, when the molecular mass of amines (R-NH2) increases, solubility declines due to the increased size of the hydrophobic alkyl component.
Because of hydrogen bonding between the nitrogen of one molecule and the hydrogen of another, primary and secondary amines form intermolecular associations. Because primary amines have two hydrogen atoms available for hydrogen bond formation, they have higher intermolecular interaction than secondary amines. Because there is no hydrogen atom available for hydrogen bond formation, tertiary amines do not exhibit intermolecular interaction. As a result, the boiling temperatures of isomeric amines are in the following order:
Primary > Secondary > Tertiary
Benzenediazonium chloride is a crystalline substance that is colourless. It is generally water (H2O) soluble and generally stable at room temperature, but when warmed, it reacts with water. In the dry state, it decomposes quickly. Water insoluble, benzenediazonium fluoroborate is stable at ambient temperature.
Amines are reactive because of the difference in electronegativity between nitrogen and hydrogen atoms, as well as the presence of an unshared pair of electrons above the nitrogen atom.
Basic Character of Amines
Basic character of amines can be explained as follows:
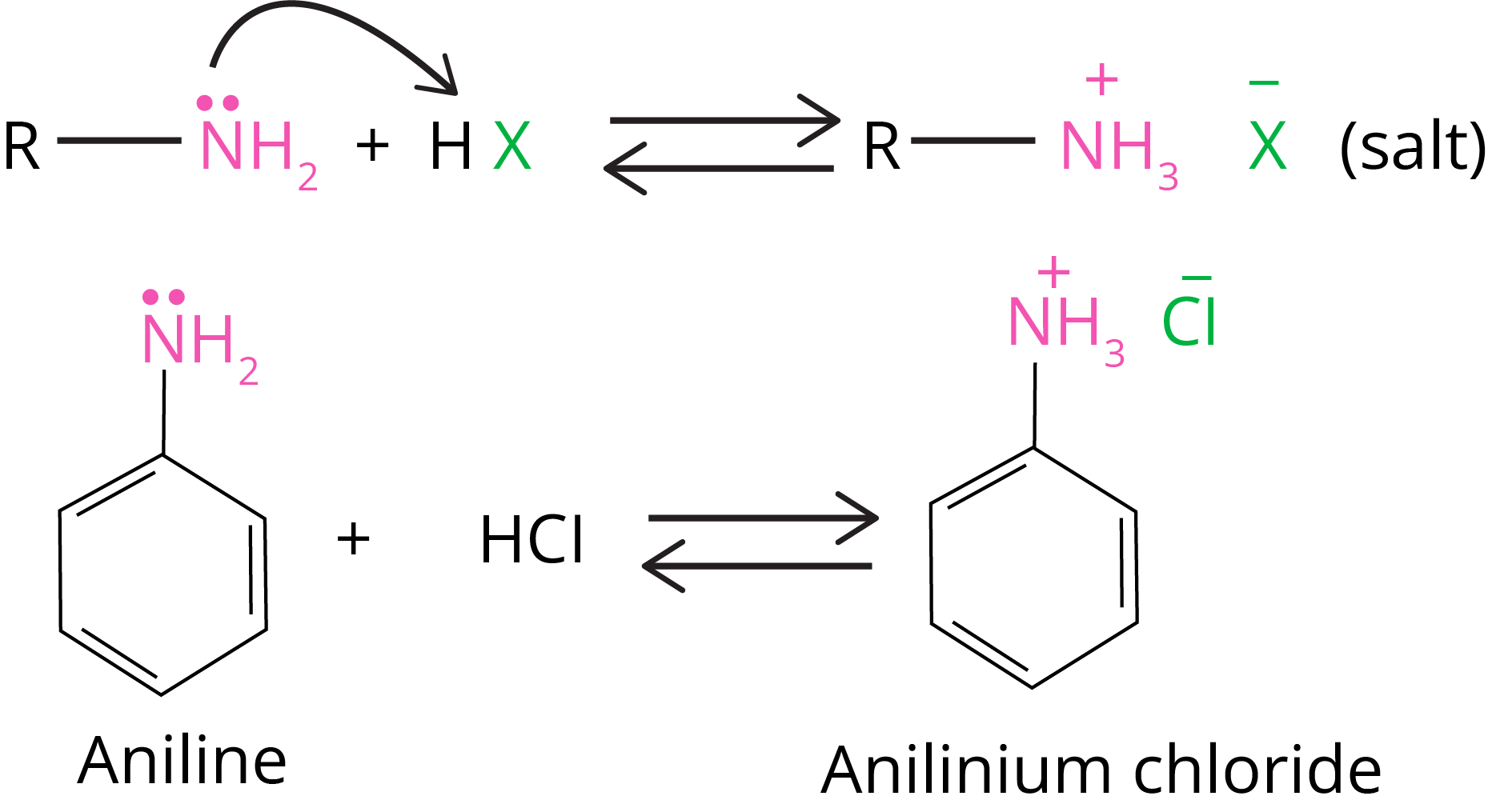
They react with acids to generate salts.
Amine salts regenerate the parent amine when treated with a base such as NaOH.
R+NH3X- + OH- →RNH2 + H2O + X-
Amines react with mineral acids to generate ammonium salts, indicating that they are basic in nature.
Amines are Lewis bases because they have an unshared pair of electrons on nitrogen atoms.
The stronger the base, the higher the value of Kb or the lower the value of pKb.
PKb= -log Kb
Preparation Reaction for Amines
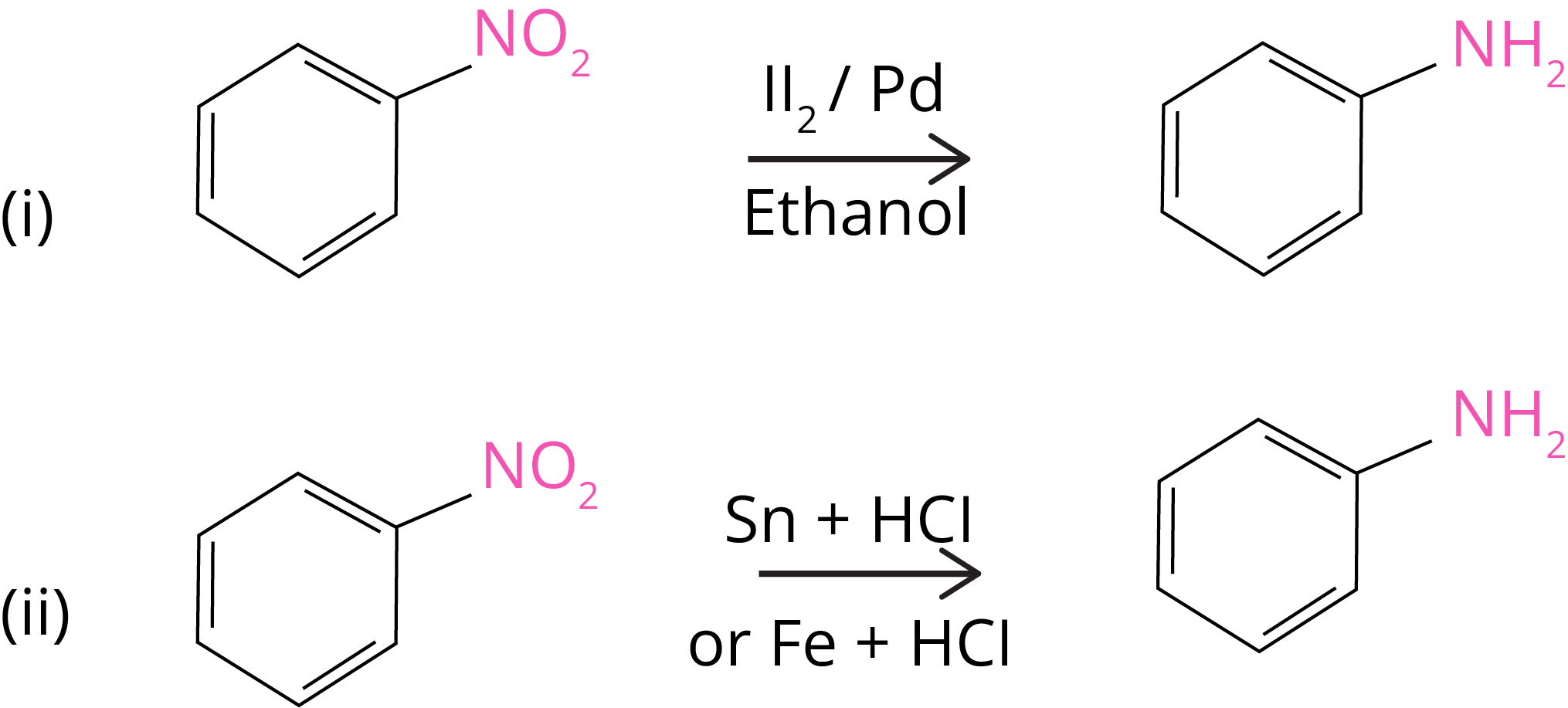
1. Reduction of Nitro Compounds
By passing hydrogen gas through finely divided nickel, palladium, or platinum, nitro compounds are reduced to amines, as well as by reduction with metals in an acidic media.
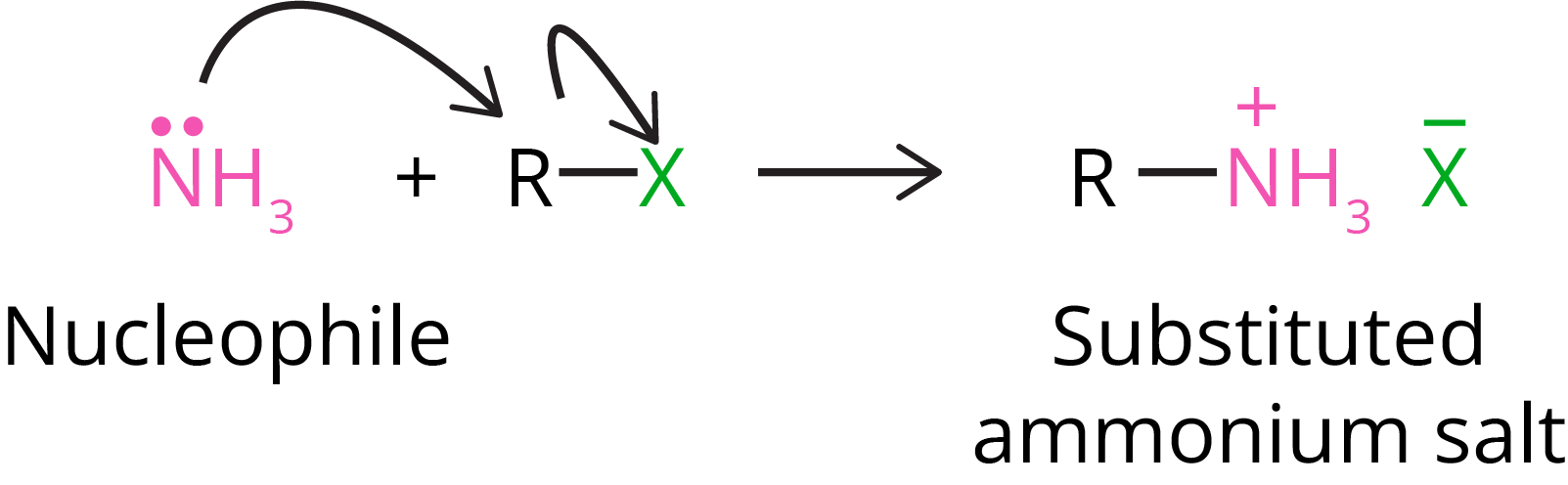
2. Ammonolysis of Alkyl Halide
A nucleophile can easily cleave carbon-halogen bonds in alkyl or benzyl halides. As a result, when an alkyl or benzyl halide reacts with an ethanolic solution of ammonia, the halogen atom is replaced by an amino (–NH2) group in a nucleophilic substitution reaction.
RNH2 + RX → R2NH
R2NH + RX → R3N
R3N + RX → R4+NX-
Treatment with a strong base yields the free amine from the ammonium salt:
R+NH3X- + NaOH → R-NH2 + H2O + NaX
3. Reduction of Nitriles
Primary amines are formed when nitriles are reduced with lithium aluminium hydride (LiAlH4) or hydrogenated catalytically.
R-CN + H2/Ni + Na(Hg)/C2H5OH → R-CH2-NH2
4. Reduction of Amides
When amides are reduced with lithium aluminium hydride, amines are formed.
R-C(O)-NH2 + LiAlH4 + H2O→ R-CH2-NH2
5. Gabriel Phthalimide Reaction
Primary amines are made via the Gabriel synthesis method. When phthalimide is treated with ethanolic potassium hydroxide, it generates a potassium salt, which when heated with an alkyl halide and then alkaline hydrolyzed yields the primary amine.
Because aryl halides do not undergo nucleophilic substitution with the anion generated by phthalimide, this approach cannot be used to make aromatic primary amines.
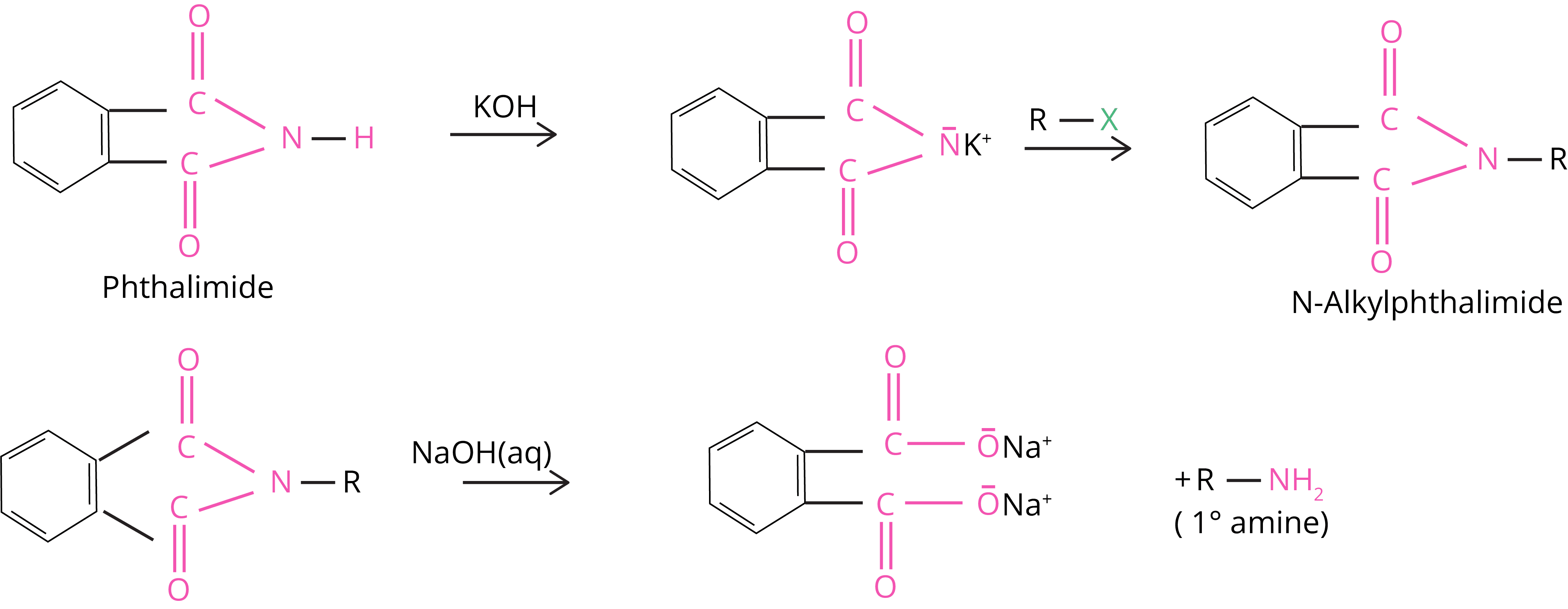
6. Hoffmann Bromamide Degradation Reaction
By treating an amide with bromine in an aqueous or ethanolic solution of sodium hydroxide, Hoffmann created a method for preparing primary amines.
R-C(O)-NH2 + Br2 + 4NaOH→ R-NH2 + Na2CO3 + 2NaBr + 2H2O
Chemical Reactions of Amine
1. Carbylamine Reaction
When primary amines are heated with chloroform and ethanolic potassium hydroxide, they generate isocyanides or carbylamines, which have a horrible odour. This reaction does not occur in secondary or tertiary amines.
R-NH2 + CHCl3 + 3KOH → R-NC + 3KCl + 3H2O (on heating)
2. Reaction with Nitrous Acid
When primary aliphatic amines react with nitrous acid, they produce aliphatic diazonium salts, which are unstable and release nitrogen gas and alcohols in large quantities.
R-NH2 + HNO2 (NaNO2 + HCl) → RN+2Cl-
RN+2Cl- + H2O → ROH + N2 + HCl
At low temperatures (273-278 K), aromatic amines react with nitrous acid to create diazonium ions.
C6H5-NH2 + HNO2 (NaNO2 + HCl) → C6H5N+2Cl- + NaCl + 2H2O
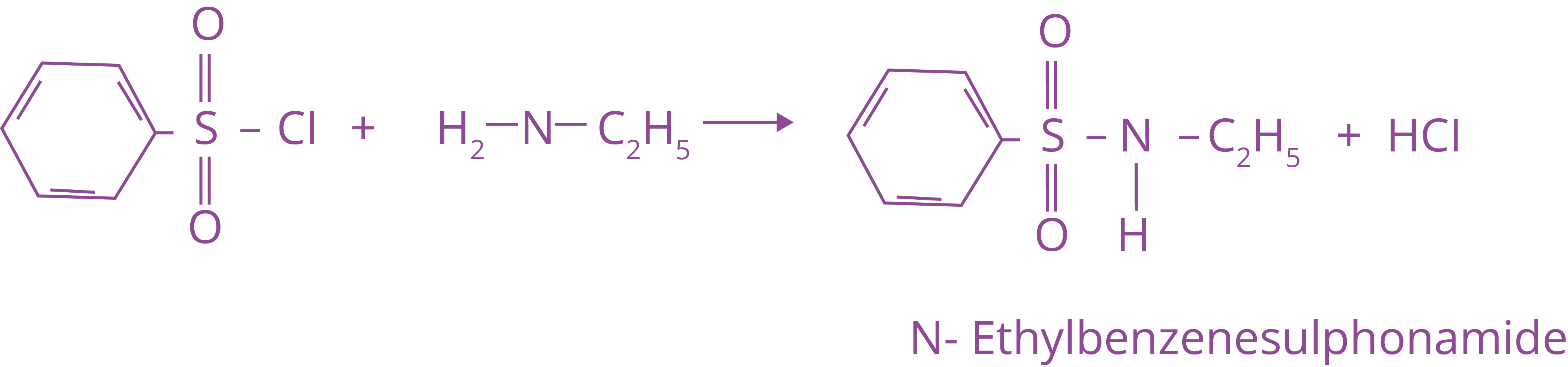
3. Reaction with Arylsulphonyl Chloride
Hinsberg's reagent, benzenesulphonyl chloride (C6H5SO2Cl), interacts with primary and secondary amines to produce sulphonamides.
(a) N-ethylbenzenesulphonyl amide is formed by reacting benzenesulphonyl chloride with primary amine.
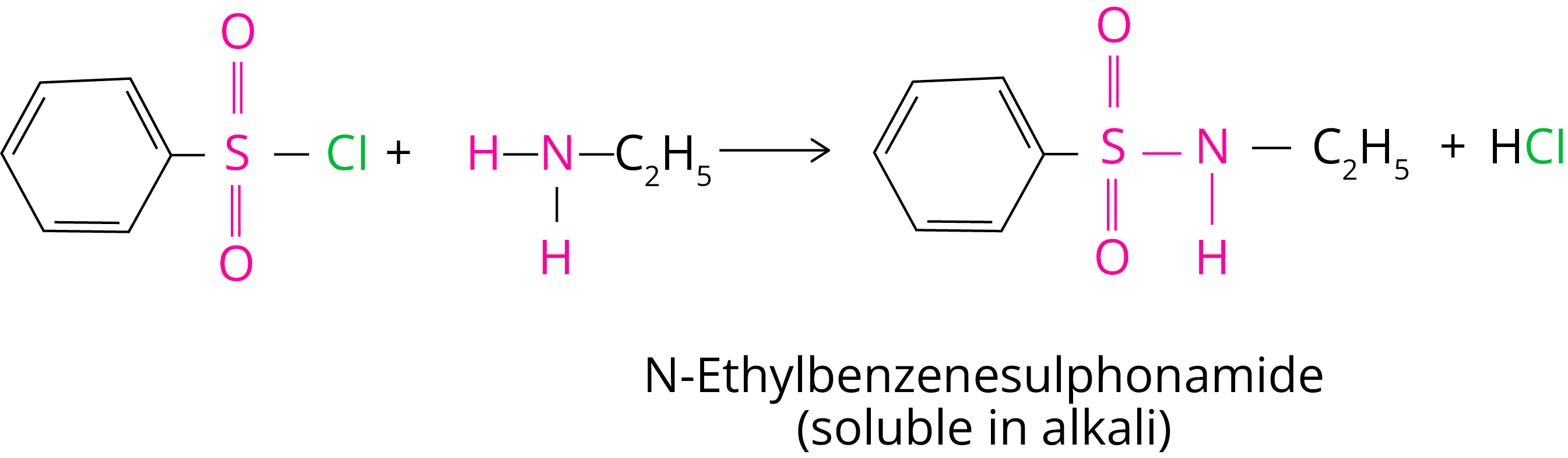
Due to the presence of a strong electron withdrawing sulfonyl group, the hydrogen linked to nitrogen in sulphonamide is extremely acidic. As a result, it is alkali soluble.
(b) N,N-diethylbenzenesulphonamide is produced in the reaction with secondary amine.
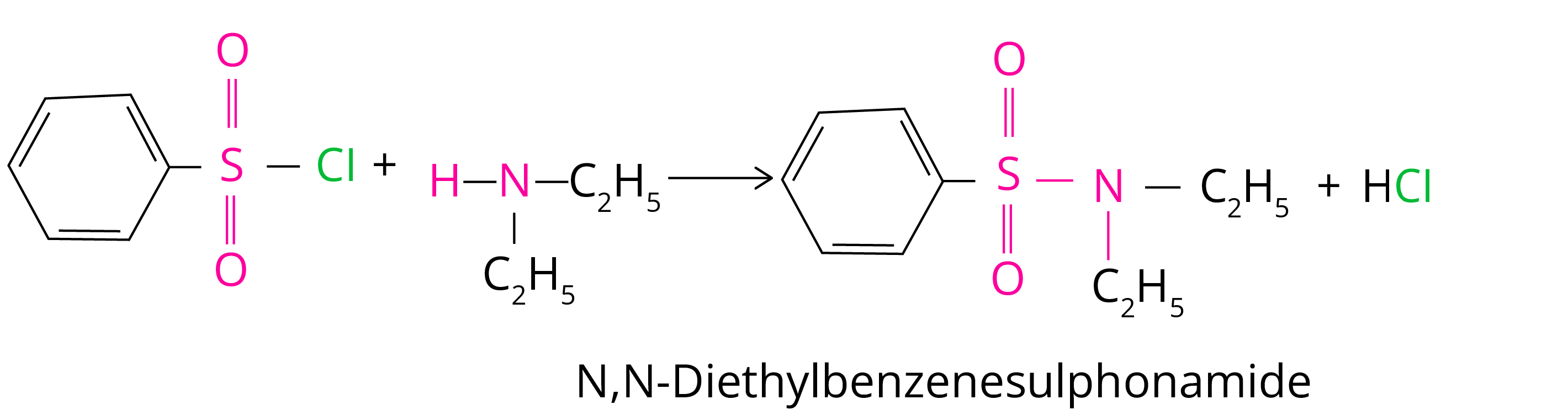
Tertiary amines do not react with benzenesulfonyl chloride.
4. Electrophilic Substitution Reaction
An ortho-para directing group is the Amine group. It is subjected to a number of electrophilic substitution processes.
(a) Bromination:
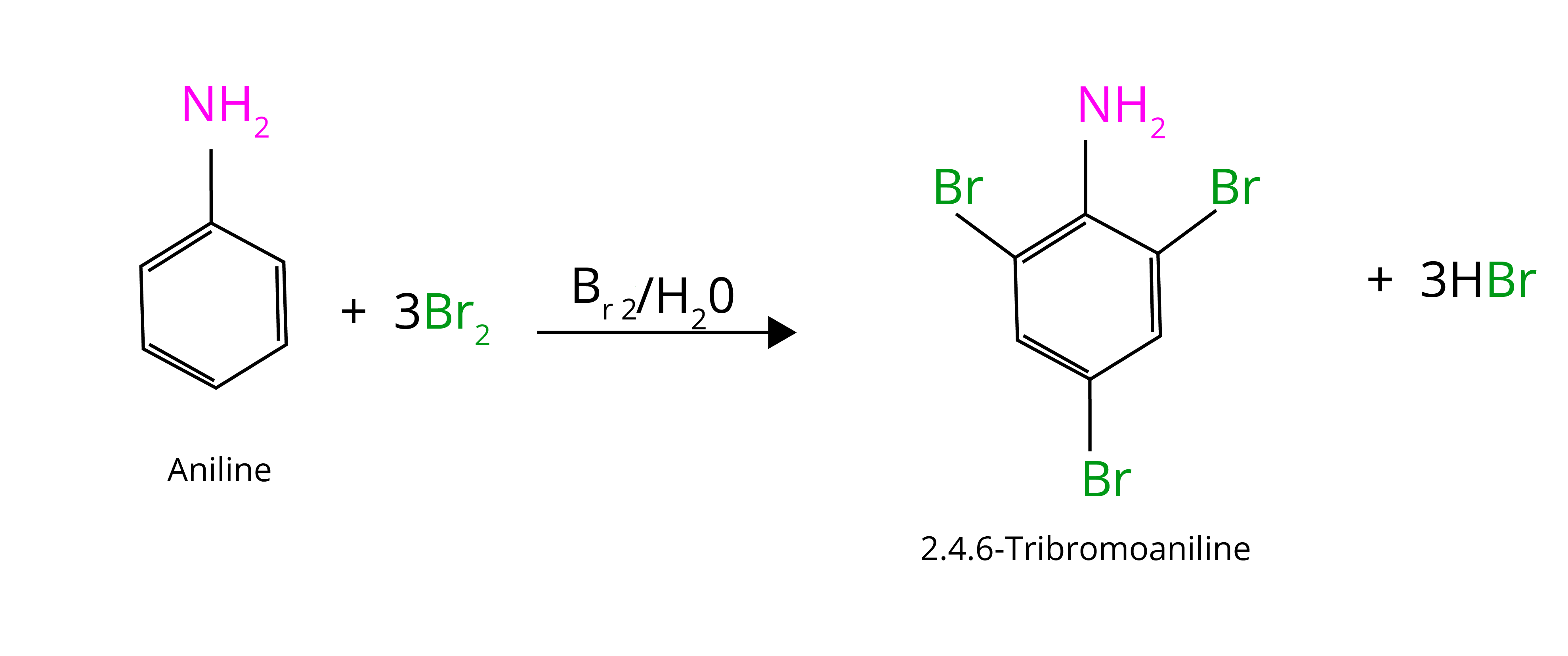
At room temperature, aniline reacts with bromine water to form a white precipitate of 2,4,6-tribromoaniline. In the presence of an acetyl group, controlled bromination can occur. It shields the amine group and keeps uncontrolled halogenation under control (bromination).
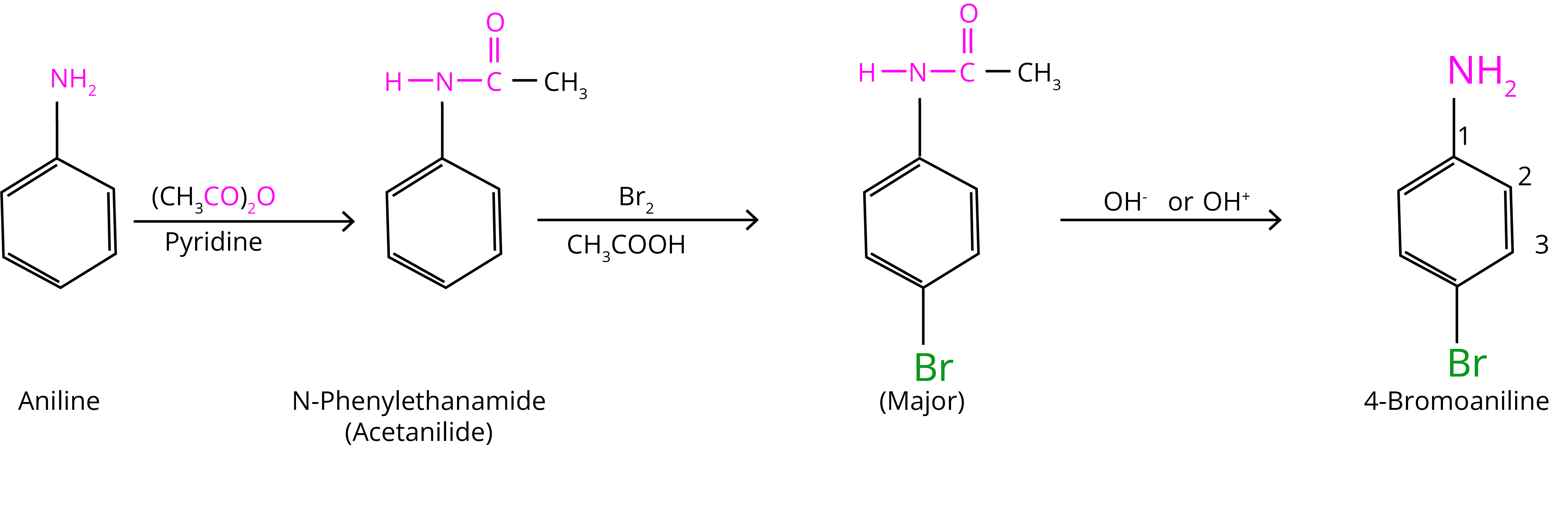
(b) Nitration
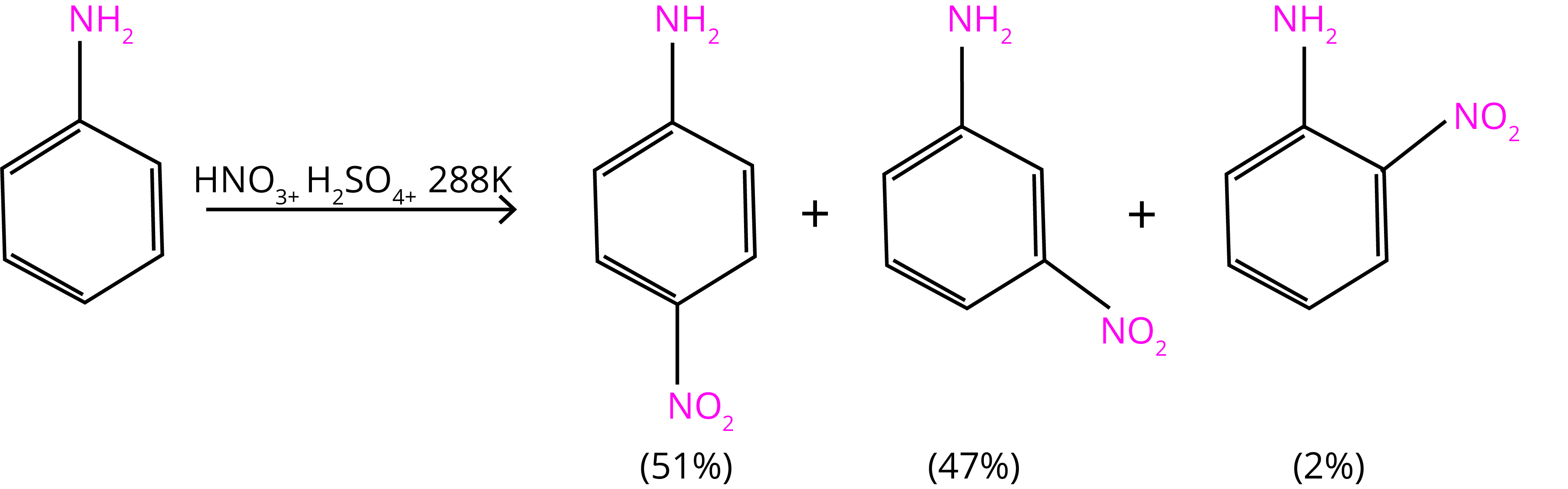
Aniline is protonated to create the meta directing anilinium ion in a very acidic solution. As a result, in addition to ortho and para derivatives, a considerable number of meta derivatives are generated.
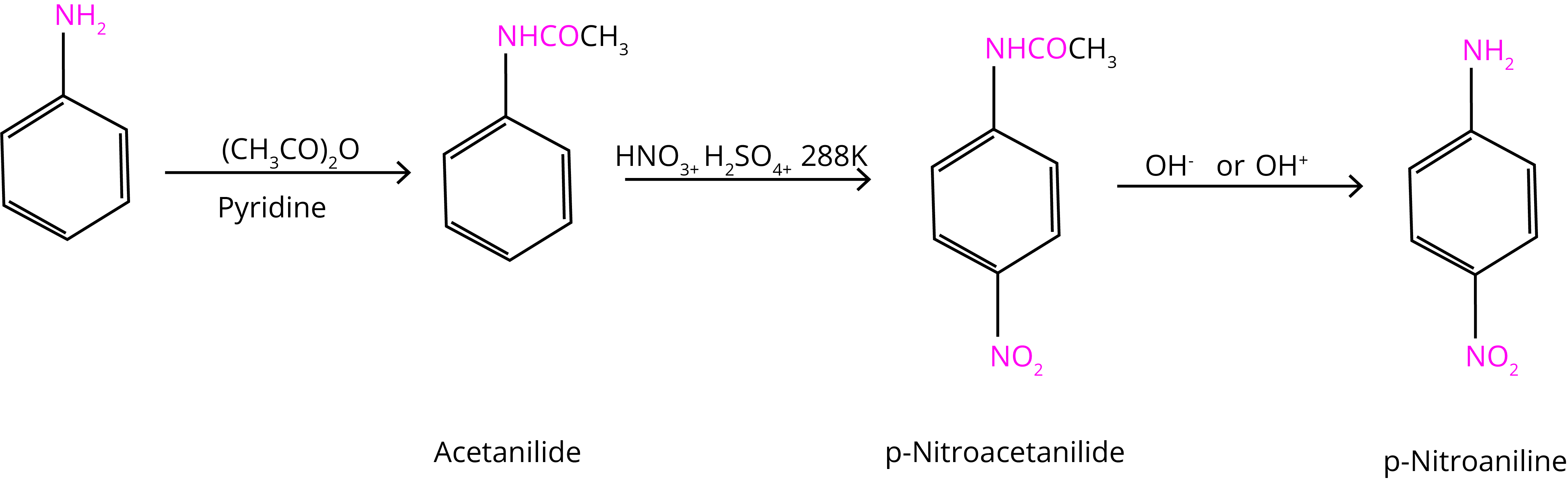
The nitration reaction can be regulated and the p-nitro derivative obtained as the primary product by protecting the –NH2 group via an acetylation reaction with acetic anhydride.
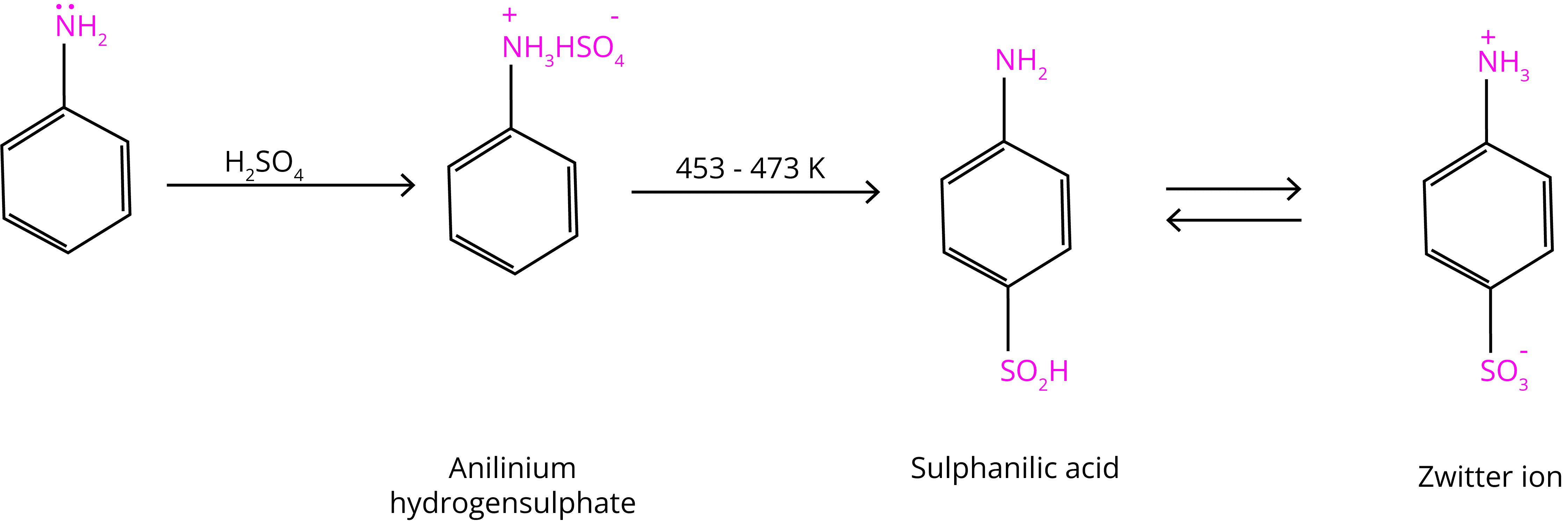
(C) Sulphonation
Aniline combines with concentrated sulphuric acid to make anilinium hydrogensulphate, which produces p-aminobenzene sulphonic acid when heated with sulphuric acid at 453-473K.
Why Aniline do not undergo Friedel Crafts Reaction
Because the Lewis acid, aluminium chloride, is utilised as a catalyst, aniline does not undergo Friedel-Crafts reaction (alkylation and acetylation). As a result, the nitrogen in aniline gains a positive charge, acting as a powerful deactivate group for subsequent reactions.
Resonance in Diazonium Salt
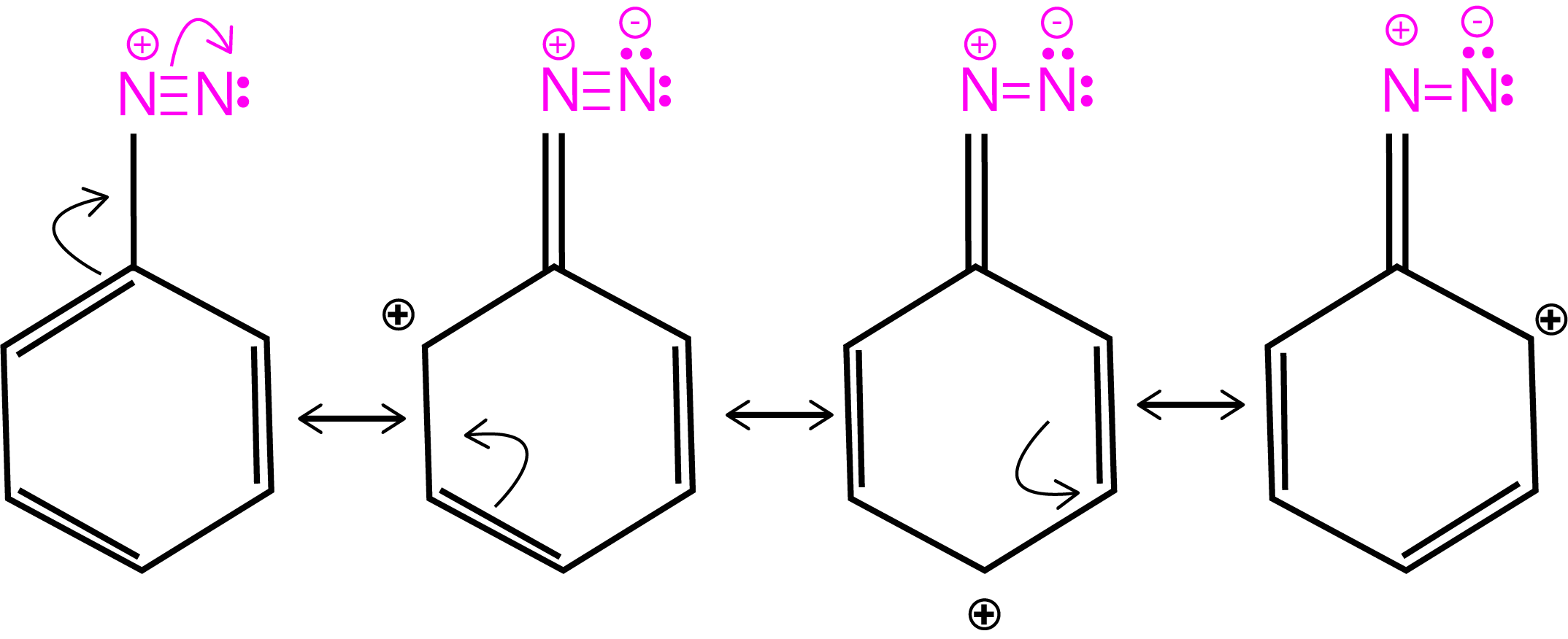
Preparation Method of Diazonium Salt
The reaction of aniline with nitrous acid at 273-278K produces benzene diazonium chloride. The reaction of sodium nitrite with hydrochloric acid produces nitrous acid in the reaction mixture. Diazotization is the process of converting primary aromatic amines into diazonium ions.
C6H5NH2 + NaNO2 + 2HCl →C6H5N+2Cl- + NaCl + 2H2O
Reactions of Diazonium Salt
1. Sandmayer’s Reaction
Replacement by halide or cyanide ion: In the presence of Cu(I) ions, the nucleophiles Cl–, Br–, and CN– can easily be introduced into the benzene ring. The Sandmeyer reaction is the name for this reaction.
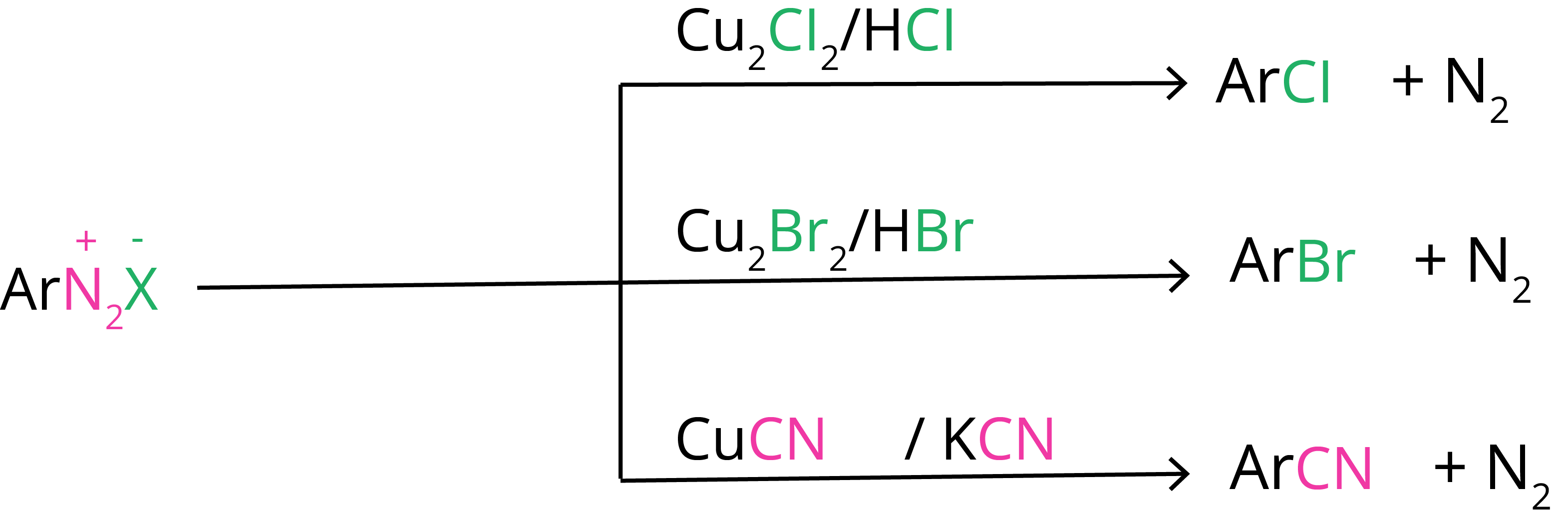
2. Gatterman Reaction
By treating the diazonium salt solution with the appropriate halogen acid in the presence of copper powder, chlorine or bromine can be introduced into the benzene ring. The Gatterman reaction is the name for this.
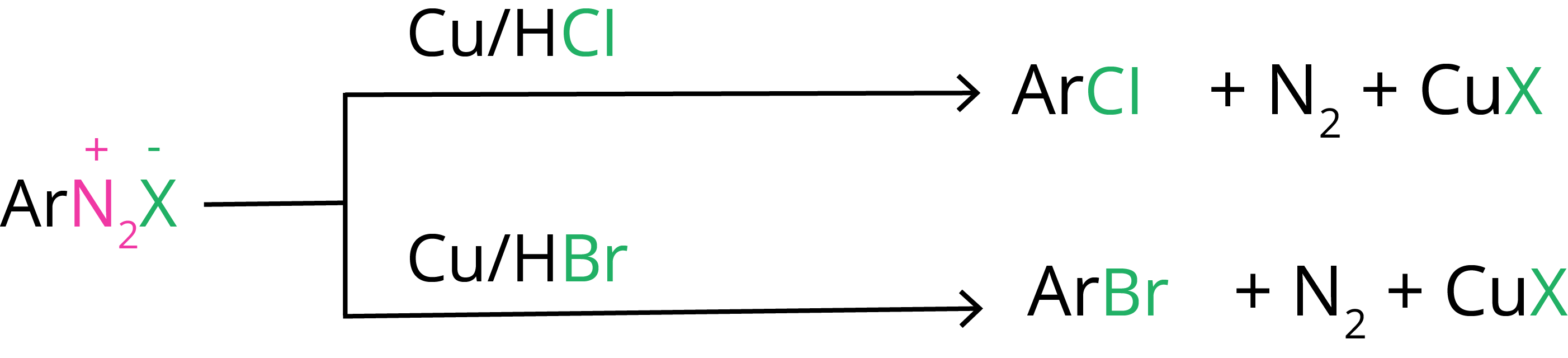
3. Replacement by Iodide Ion:
ArN+2Cl- + KI → ArI + KCl + N2
4. Replacement by Fluoride ion:
ArN+2Cl- + HBF4 → Ar-N+2BF-4
Ar-N+2BF-4 → Ar-F + BF3 + N2
5. Replacement by H:
ArN+2Cl- + H3PO2 + H2O → ArH + N2 + H3PO3 + HCl
ArN+2Cl- + CH3CH2OH → ArH + N2 + CH3CHO + HCl
6. Replacement by Hydroxyl Group:
ArN+2Cl- + H2O → ArOH + N2 + HCl
7. Replacement by –NO2 Group:
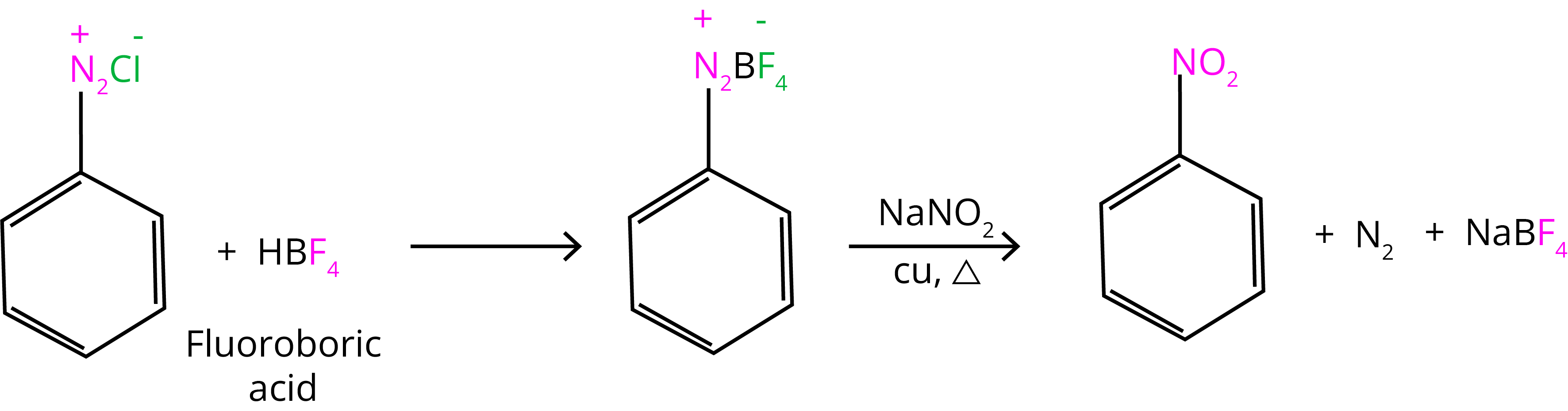
8. Coupling Reaction:
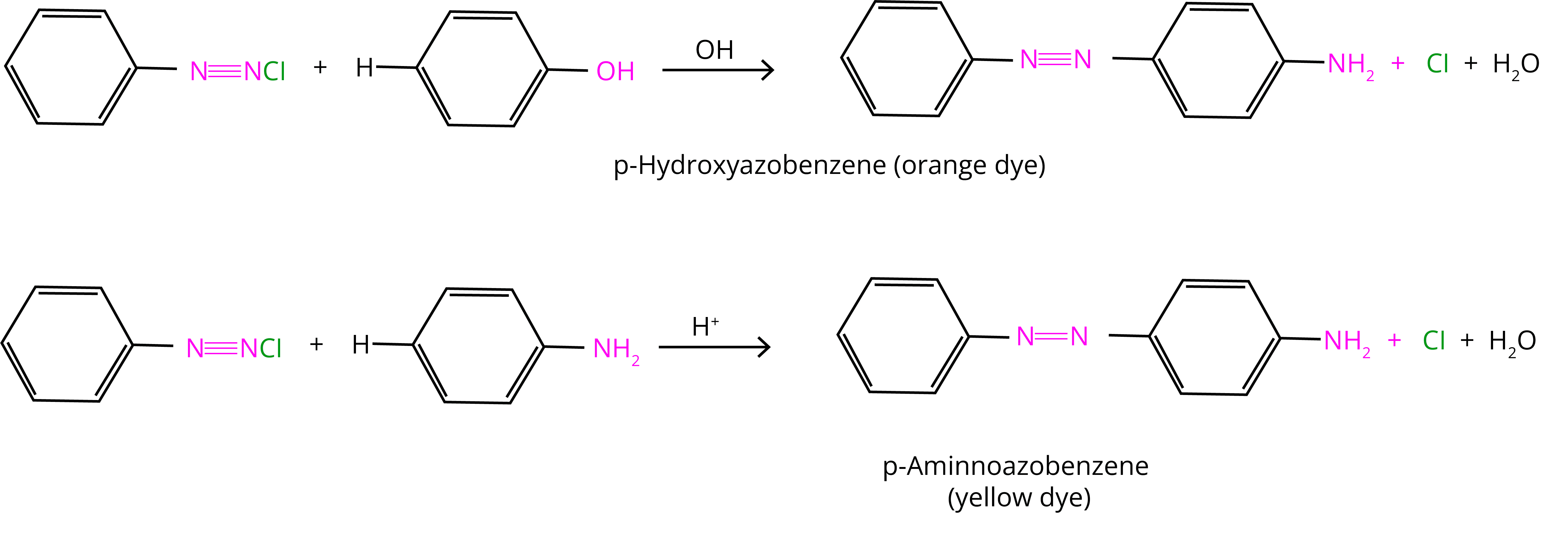
Solved Examples/Problems from the Chapter: Amines
1. How will you convert: benzene into aniline
Ans: 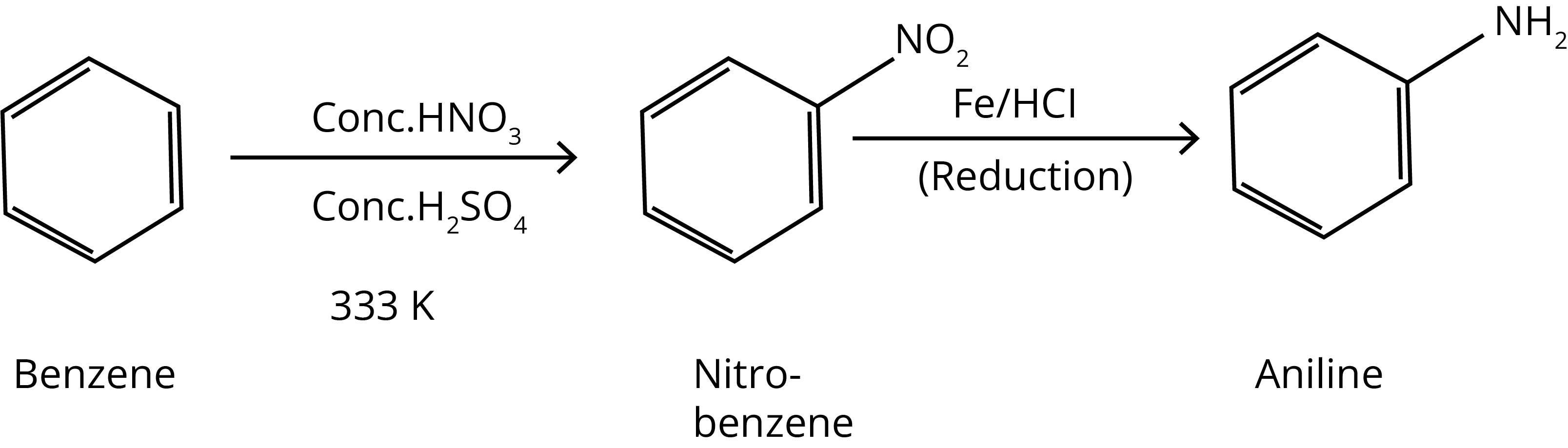
Key Point: Nitro group can be converted into amine group with the help of reducing agent.
2.How will you convert: Cl-(CH2)4-Cl into Hexane -1,6- diamine
Ans: 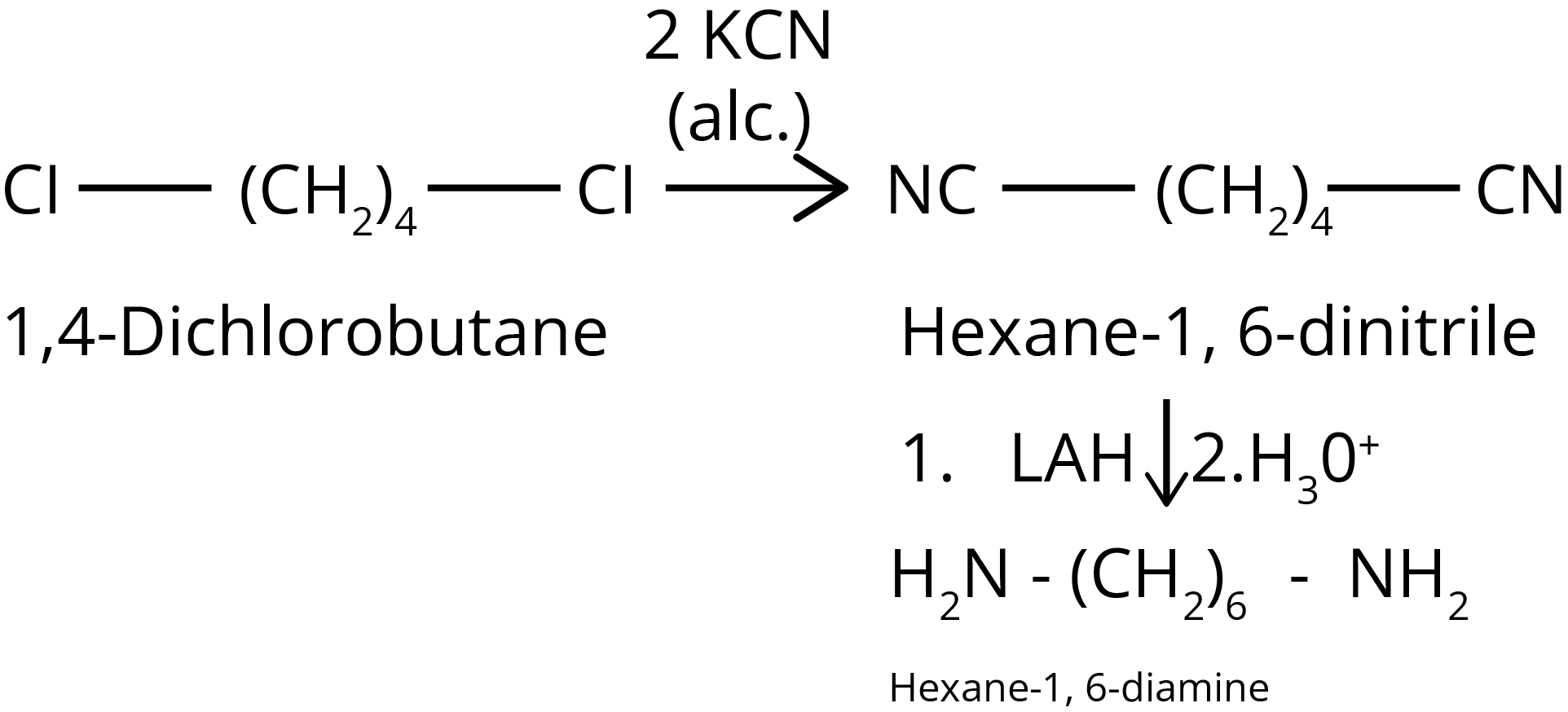
Key Point: Cyanide group can be converted into amine group with the help of reducing agent. Cyanide group increases one carbon in the parent chain.
Solved Problems of Previous Year Question From the Chapter: Amines
1. Identify the compound that will react with Hinsberg’s reagent to give a solid which dissolves in alkali.
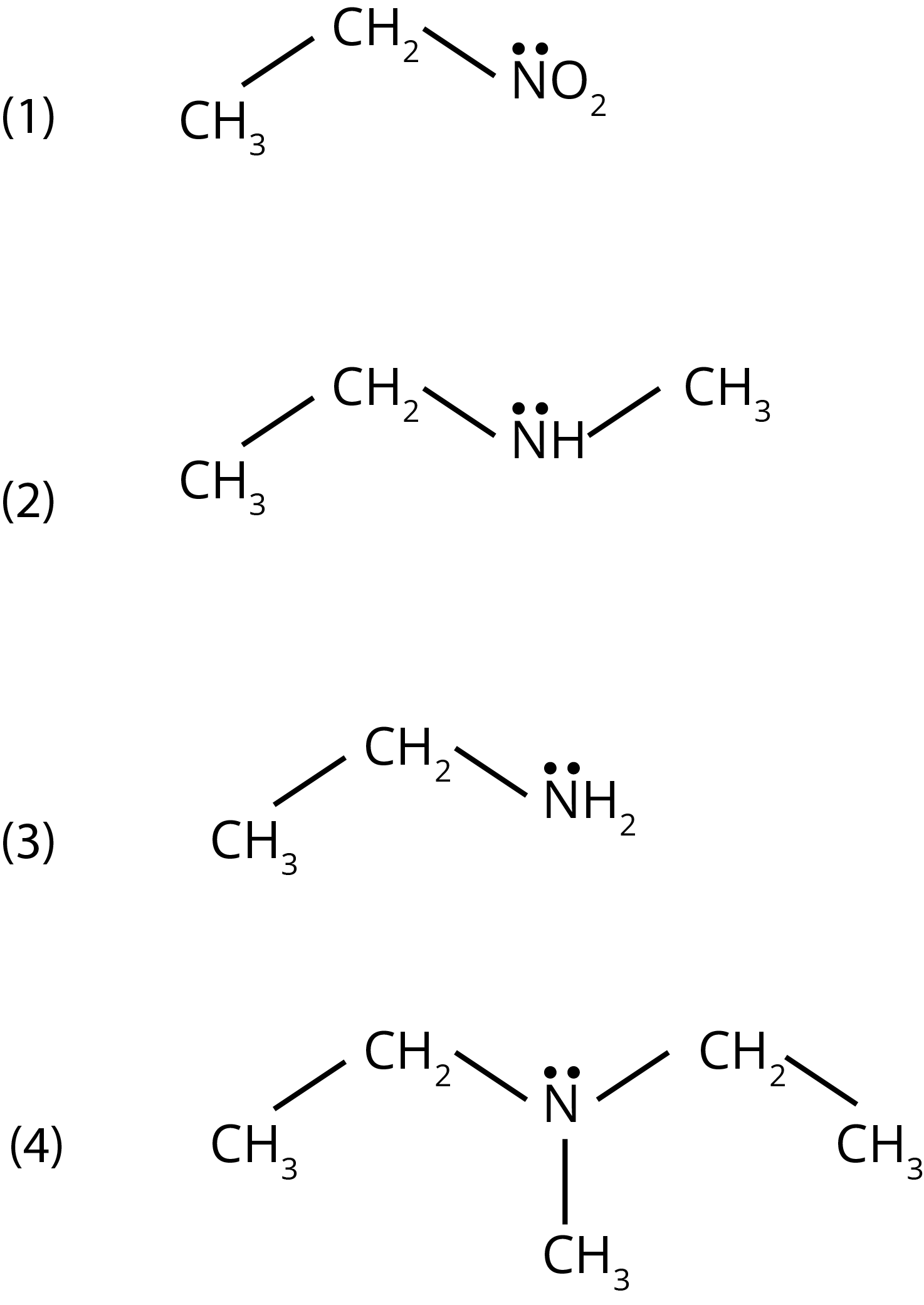
Ans: The correct answer is option 3. Only primary amines react with hinsberg reagents and forms precipitate that dissolve in addition to alkali.
Trick: Hinsberg test is used to distinguish between the amine. Primary and secondary amine give this test (product formed with primary amine is soluble in alkali while with secondary amine is insoluble in base) and tertiary amine do not give this test.
2. Which of the amines will give a carbylamine test?
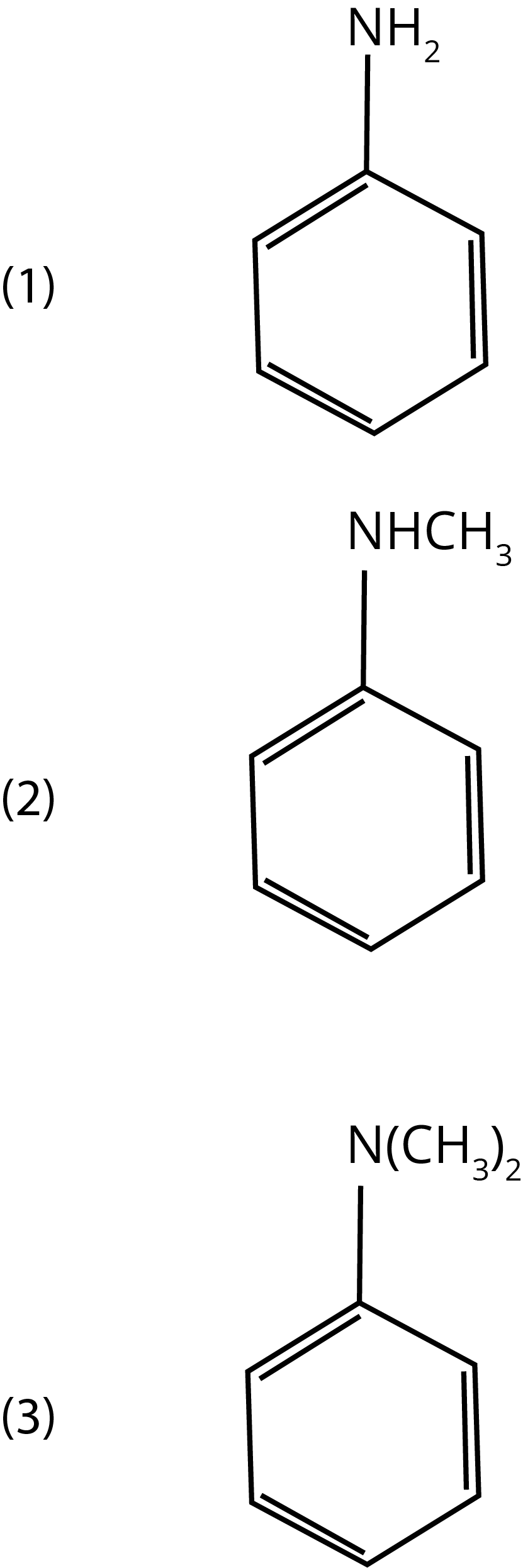
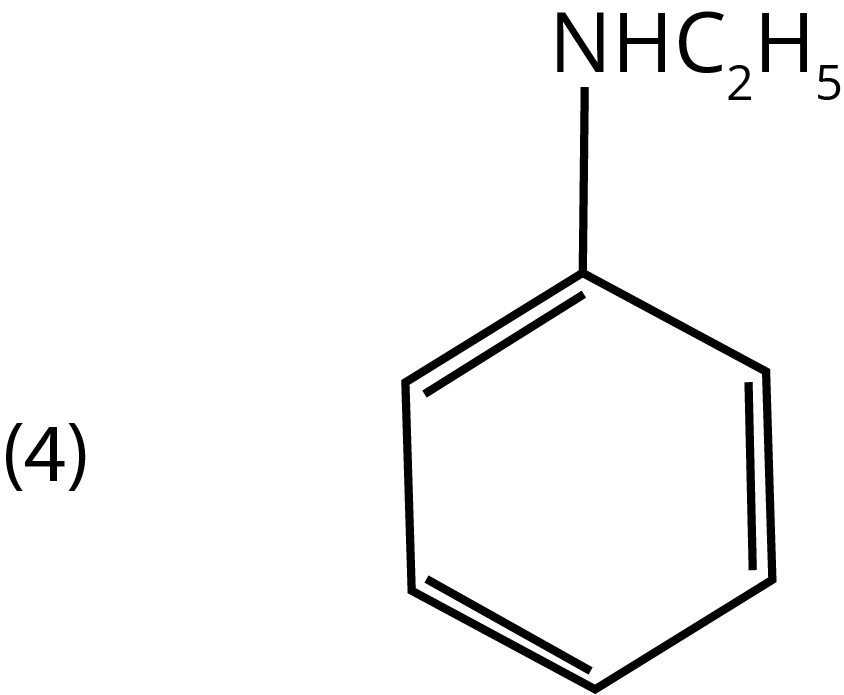
Ans: The correct answer is option 1. Carbylamines (or isocyanides) are formed when aliphatic/aromatic primary amines are heated with chloroform and alcoholic KOH.
Ph-NH2 + CHCl3 + 3KOH → Ph-NC + 3KCl + 3H2O
Trick: This test is not given by secondary or tertiary amines.
3. The reagent ‘R’ in the given sequence of chemical reaction is :

(1) H2O
(2) CH3CH2OH
(3) HI
(4) CuCN/KCN
Ans: The correct answer for this question is option 2.

Trick: Some weak reducing agents, such as hypophosphorous acid or ethanol, convert diazonium salts to arene, which is then oxidized to phosphorous acid or ethanal.
Practice Questions
1. Convert: 3 - Methylaniline into3 - nitrotoluene
Ans:
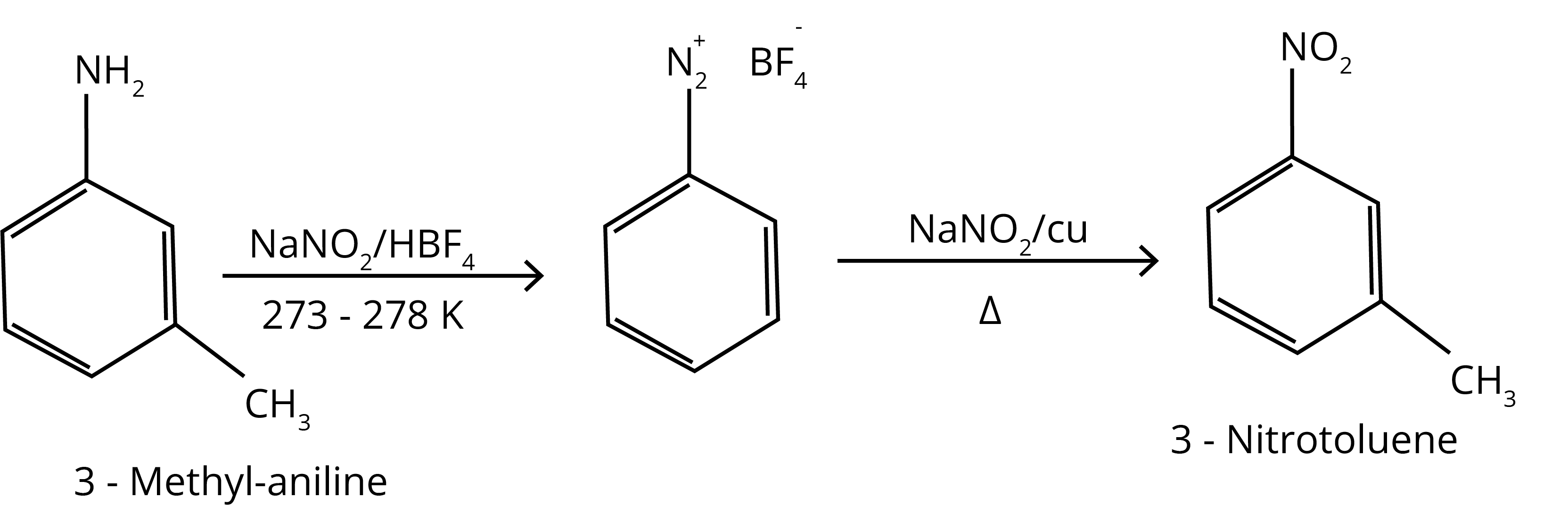
2. Why Diazonium salts of aromatic amines are more stable than those of aliphatic amines.
Ans: Due to resonance
Conclusion
In this amine's notes we have provided important information regarding the chapter compounds of nitrogen such as important definitions, reactions, and concepts. Students should work on more solved examples and organic compounds of nitrogen examples for securing good grades in the NEET exams.
NEET Chapter - Amines

 Share
ShareFAQs on NEET Chapter - Amines
1. What are the key factors to remember before taking the NEET exam and answering questions about amines?
To be more clear with the concepts, students should practise writing the mechanisms of various reactions that are included in this chapter. Students can also improve their grades by practicing resonating structure and previous year problems. Amine is an important chapter for NEET exam and thus one must practice all the formulas and chemical reactions thoroughly to score good marks in this section.
2. Do questions from the amine chapter come every year in NEET?
Yes, the amine chapter is one of the important chapters for the NEET and thus, several questions are asked in the NEET exam every year. This chapter is considered a scoring chapter because students can get full marks for all the correct and to the point equations. The topics are concept based and thus requires direct answers. The important topics of this chapter are Amine preparation, Amino acid basicity and stability of amines.
3. What is the weightage of this chapter in the NEET exam?
Nearly 1-2 questions are asked in the NEET exam from this chapter. The chapter covers 4 marks in the NEET exam which makes about 2% of the total marks. Some of the important topics from this chapter that the students must focus upon are Amino acid basicity, stability of amines and Amine preparation.




















 Watch Video
Watch Video


