




How Is Tritium Used in Daily Life and Science?
Tritium is an isotope (an isotope is one of two or more forms of the same element) of hydrogen. It is also called Hydrogen-3. Tritium is commonly denoted by the letter T or ³H. It is extremely rare. It is also the only isotope of hydrogen that is radioactive. It has one proton and two neutrons which makes it three times heavier than an ordinary hydrogen atom.
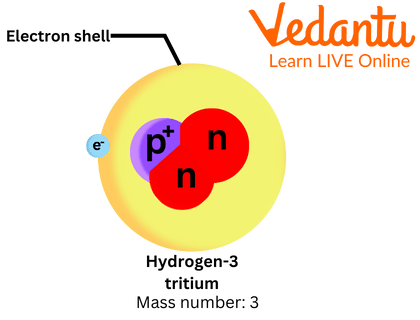
Structure of Tritium Atom
Tritium emits only beta radiation. It is relatively safe as it does not penetrate human skin. Tritium can exist as an odourless and colourless gas like ordinary hydrogen but it is primarily found in liquid form as part of tritiated water, a form of heavy water.
Applications of Tritium
Tritium can be used to make self-sufficient light sources. The electrons emitted by small amounts of tritium can cause the chemicals called phosphors to glow. This phenomenon is used in self-powered lighting devices. These are now used in watches and exit signs. These lights are not bright, but they are useful for illuminated signs and firearms sights for nighttime use. It is also used for making glowing keychains and compasses.

Self-Powered Lighting
It is also used in emergency lighting in buildings and lights on the airport runway.

Tritium in Exit Signs
Tritium is used as a source of energy for thermonuclear weapons. In this application, tritium and deuterium atoms are combined in a nuclear fusion reaction, which releases a tremendous amount of energy.
Tritium is also used as a tracer for the investigation of chemical structures and reactions. A tracer acts the same way that the ordinary atoms of the element behave.
It is also used in hydrological studies to trace geological water and the movement of glaciers.
It is also used in night vision devices.
Tritium Uses in Medicine
In medicine, tritium is used for diagnosis and radio treatment.
In Biology, it is used to mark hydrogen and study its metabolism.
Learning by Doing
Choose the Correct Answer.
1. Protium and deuterium are more common isotopes of hydrogen compared to tritium.
True
False
2. Which radiation does a tritium atom emit?
Alpha
Beta
Gamma
It does emit any radiation.
Solved Questions
1. Give two uses of hydrogen-3.
Ans: Two uses of hydrogen-3 are listed below:
In medicine, it is used for diagnosis and radio treatment.
Tritium is used as a source of energy for thermonuclear weapons.
2. How is tritium used in hydrological studies?
Ans: In hydrological studies, tritium is used to trace geological water and the movement of glaciers.
Summary
The tritium nucleus is not stable and decays into helium-3 by emitting beta radiation (electrons). The half-life (the amount of time it takes for one-half of a radioactive isotope to decay) of this tritium is 12.3 years. Each year 5.5% of the tritium decays to helium-3.
FAQs on Uses of Tritium: Key Applications & Concepts
1. Who discovered tritium?
Physicists Ernest Rutherford, M.L. Oliphant, and Paul Harteck discovered tritium in 1934. They obtained the isotope from a sample of deuterium but were unable to isolate it. Luis Alvarez and Robert Cornog isolated tritium in 1939.
2. What are the health risks of tritium?
Tritium poses no danger to humans or animals externally because it is a low-energy beta emitter. However, it poses a radiation danger when inhaled, injected, consumed, or absorbed through the skin. The main health risk associated with beta exposure is a high risk of cancer.
3. What is the atomic mass of tritium?
The atomic mass of tritium is 3.016u.









