




An Overview of Class 9 Biology Osmosis In Raisins Experiment
Have you ever observed how some seeds or dry fruits like raisins or apricots swell underwater? The raisins increase in size and become more rounded, contrary to their shrivelled appearance in the absence of water. Raisins are just that- shrivelled, dehydrated grapes! Why did the raisins increase in their size? This is because the raisins have carried out a process called osmosis. Carry out the following osmosis experiment to understand what happens during the process!
Table of Content
Aim
Apparatus required
Theory
Procedure
Observations
Result
Precautions
Lab manual questions
Viva questions
Practical based questions
Aim
To find out the mass percentage of water imbibed by raisins.
Apparatus Required
Raisins (10-15), Petri dish or beaker, distilled water, digital balance, blotting paper
Theory
A cell must be able to allow the transport of different substances in and out of its boundaries. This transport occurs through diffusion and osmosis.
Osmosis is the net diffusion of solvent molecules from an area of high water potential to an area of lower water potential through a semipermeable membrane.
Osmosis continues until there is an equal solute concentration on both sides of the membrane.
Exosmosis occurs when water moves out from a cell. It makes a cell flaccid. This leads to the shrinking away of the plasma membrane from the cell wall, a process called plasmolysis.
The process of endosmosis occurs when water moves into a cell. It makes the cell turgid.
Raisins become turgid and swell in size when soaked in water. This occurs due to the imbibition of water and endosmosis.
One can determine the percentage of water imbibed by the raisins through the following formula:
\[{\rm{ }}\dfrac{{{\rm{weight of water absorbed by raisins}}}}{{{\rm{weight of dry raisins }}}} \times 100\]
Procedure
Take the raisins in a watch glass and measure their weight using a digital balance.
Place the raisins into a Petri dish or beaker with distilled water, and cover it with its lid.
Let the raisins soak in the water overnight.
Next day, take the resins out of the water and place them onto a blotting paper to get rid of the excess water. Pat them dry by gently pressing the second blotting paper over them.
Weigh the swollen raisins and record their weight.
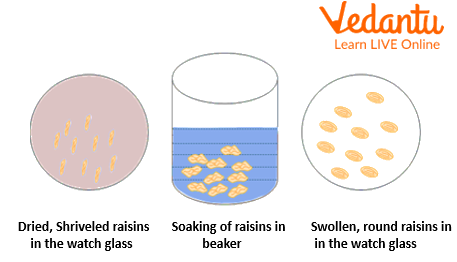
Osmosis in raisins
Observations and Calculations
The raisins appear larger and have become rounded in appearance. This is because of endosmosis, whereby raisins take water in a hypotonic solution.
Weight of dry raisins (w1): ________ grams
Weight of swollen raisins (w2): ___________ grams
Weight of water imbibed by raisins (i.e. w2-w1): _______ (w grams)
Percentage of water imbibed by raisins:
\[ = \dfrac{{{\rm{weight of water absorbed by raisins }}}}{{{\rm{weight of dry raisins }}}} \times 100\]
\[ = \dfrac{{{w_2} - {w_1}}}{{{w_1}}} \times 100\]
= ______ %
Results
The percentage of water imbibed by the raisins is ______ %
Precautions
The raisins must be dried, and their stalks must be intact.
The weighing before and after soaking must be done carefully to avoid errors.
The excess water from the raisins must be dried properly using blotting paper.
The raisins must be completely soaked under water so that enough water surrounds each raisin for osmosis.
Lab Manual Questions
1. What is endosmosis?
Ans. Osmosis is the movement of solvent molecules from a region of low solute concentration to a region with high solute concentration through a semipermeable membrane. It is of two types- Endosmosis and Exosmosis. Endosmosis is the process by which a cell takes up water via osmosis and swells in size.
2. What is a hypertonic solution?
Ans. A solution wherein the concentration of the solute is higher outside the cell, as compared to the concentration of solute within the cell. Such a solution is called a hypertonic solution.
3. How will imbibition vary with the surface area?
Ans. Imbibition is directly proportional to the surface area of a cell or any other imbibant. Hence, as the surface area of the imbibant increases,more imbibition of the liquid will take place.
4. Give reasons- During monsoons, wooden doors do not open or close easily.
Ans: Imbibition is a process wherein water or any liquid gets absorbed by solid objects which increases the size of that solid object. This phenomenon occurs on wooden doors during monsoons. The moisture content in the atmosphere is high in monsoon, as a result water gets imbibed on the wooden surfaces of doors and windows. Due to this, there is an increase in the size of the doors. Therefore,closing and opening of the doors becomes difficult.
Viva questions
1. What do you think will happen if the swollen raisins are placed in a concentrated sugar solution?
Ans. A concentrated sugar solution is hypertonic. Hence, the raisins lose water due to exosmosis when placed in a concentrated solution, causing them to shrink.
2. What do you understand by the terms hypertonic and hypotonic?
Ans. A hypertonic solution has a higher concentration of solutes than the cell which is placed in it. On the other hand, a hypotonic solution has a lower amount of dissolved solutes in it, compared to the cell.
3. What is the difference between diffusion and osmosis?
Ans. Diffusion is simply the movement of any kind of molecule across a concentration gradient. It can occur in any of the three phases- solid, liquid or gas, and does not require a semipermeable membrane. Osmosis, on the other hand, is the movement of solvent molecules, across a semipermeable membrane, from its higher concentration to lower concentration, or from a more dilute solution to a more concentrated solution.
4. Give some examples of osmosis occurring in plants.
Ans. Osmosis in plants occurs in the roots. This is essential for the uptake of water from the soil by the plant. Osmosis also facilitates the opening of the stomatal guard cells present on the underside of the leaves.
5. How is imbibition useful to plants?
Ans. Imbibition helps plants absorb water from the soil via the root cells. It also helps in maintaining the turgor pressure within plant cells. This is necessary for the maintenance of plant structures and protects them from wilting. Furthermore, turgor pressure is the key player in the opening and closing of the stomata.
6. Do you think it's possible to shrink back the raisins? How will it be done?
Ans. The swollen raisins can be placed in a concentrated salt/ sugar solution, i.e., in a hypertonic solution. This will cause the water from the raisins (higher concentration) to diffuse into the surrounding sugar or salt solution.
7. How will you deplasmolyse a cell?
Ans. A cell can be de-plasmolysed by placing it in a hypotonic solution, which has a higher concentration of water than the cell.
8. How is osmosis applied to leeches?
Ans. Salt is often used to kill leeches. Applying salt onto a leech causes osmosis, leading to water loss from the animal's cells, thereby killing it.
9. Name the factors which affect the process of imbibition.
Ans: Imbibition is affected by factors such as:- Temperature, Pressure, pH and Tonicity.
10. Which plant cell components are imbibants?
Ans: Plant components such as starch, cellulose, proteins, pectin and lignin cause imbibition.
Practical based questions
1. The percentage of water absorbed by raisins when the weight of dry raisins is 15 grams, and the mass of water absorbed by the resins is 3 grams is
20%
25%
18%
30%
Ans: A) 20%
2. The formula w2-w1/ w1 X 100 is used to calculate
Water absorbed in a hypertonic solution
Water lost in a hypotonic solution
Water absorbed in a hypotonic solution
Water lost in a hypertonic solution
Ans: Water absorbed in a hypertonic solution
3. Plasmolysis occurs when cells are placed in a
Isotonic solution
Hypertonic solution
Hypotonic solution
Mediatonic solution
Ans: B) Hypertonic solution
4. Cells become turgid when
Medium surrounding the cell has a higher solvent concentration
Medium surrounding the cell has a lower solvent concentration
The medium surrounding cell has a lower solute concentration
Both A and C
Ans: Both A and C
5. If two separate beakers, each containing raisins immersed in water, are maintained at different temperatures, what is the most likely observation?
The raisins in the beaker at a higher temperature will imbibe more water
The raisins in the beaker at a lower temperature will imbibe more water
The raisins in both beakers will imbibe equal proportions of water
The raisins in the beaker at a lower temperature will be more swollen
Ans: The raisins in the beaker at a higher temperature will imbibe more water
6. Osmosis occurs across a
Freely permeable membrane
Impermeable membrane
Semi-permeable membrane
Semi-permeable cell wall
Ans: Semi-permeable membrane
7. What is the mass of dried raisins if the percentage of water imbibed by the raisins is 66.6%, and the weight of water absorbed by the resins is 25g?
24 grams
15 grams
18 grams
16 grams
Ans: 15 grams
8. Raisins are dried form of______
Orange
Apricot
Grapes
Sugarcane
Ans: Grapes
9. Which of the following is not required in the experiment of imbibition of raisins?
Raisins
Digital weighing balance
Distilled water
Semi-permeable membrane
Ans: Semi-permeable membrane
10. Which of the following have the lowest imbibing capacity?
Proteins
Cellulose
Pea seeds
Starch
Ans: Cellulose
Conclusions
The raisins swell up when placed in water. This is because they have each undergone endosmosis, whereby water has moved into the raisins from the surroundings (the beaker), from a region of higher concentration to that of lower concentration.
FAQs on Class 9 Biology Osmosis In Raisins Experiment
1. What are the different kinds of osmosis?
Osmosis is of two kinds- exosmosis and endosmosis. In the case of plant cells, exosmosis is said to occur when a cell loses water and plasmolysed, while endosmosis occurs when a cell takes up water and becomes turgid.
2. What are the factors that affect osmosis?
In biological membranes, osmosis is affected by the presence of membrane transport proteins such as the aquaporins, the temperature, the water potential gradient, and the cell's surface area.
3. What is reverse osmosis?
When water molecules move across a semipermeable membrane against the natural osmotic concentration gradient, the process is said to be reverse osmosis. Reverse osmosis is often applied to water purification.





































