Hydrogen - Free PDF Download
Free PDF download of NCERT Exemplar for Class 11 Chemistry Chapter 9 - Hydrogen solved by expert Chemistry teachers on Vedantu.com as per NCERT (CBSE) Book guidelines. All Chapter 9 - Hydrogen Exercise questions with solutions to help you to revise complete syllabus and score more marks in your Examinations.
Question: How to download NCERT Exemplar for Class 11 Chemistry Chapter 7 Redox Reactions?
Answer: Students can download NCERT Exemplar for Class 11 Chemistry Chapter 7 Redox Reactions at Vedantu’s official website.
Hydrogen is the first and a very special element of the periodic table. It is the lightest atom on earth with just a single electron. With its 3 stable isotopes being protium, deuterium, and tritium, Hydrogen is the most abundant element in the universe.
Class 12 students can easily bring full marks from this Chapter by solving NCERT Exercises thoroughly and solving all questions from previous year papers. This will help them to create an Exam-like situation and assist them in breaking down the question-asking pattern.
Access NCERT Exemplar Solutions for Grade 11 Chemistry Chapter 9. - Hydrogen
1. Hydrogen resembles halogens in many respects for which several factors are responsible. Of the following factors, which one is most important inthis respect?
(a) Its tendency to lose an electron to form a cation.
(b) Its tendency to gain a single electron in its valence shell to attain stable electronic configuration.
(c) Its low negative electron gain enthalpy value.
(d) Its small size.
Ans: Its tendency to gain a single electron in its valence shell to attain stable electronic configuration.
Like halogens (with ns2np5 configuration belonging to the seventeenth group of the periodic table), it is short by one electron to the corresponding noble gas configuration, helium (ls2).
2. Why does H+ ion always get associated with other atoms or molecules?
(a) Ionisation enthalpy of hydrogen resembles that of alkali metals.
(b) Its reactivity is similar to halogens.
(c) It resembles both alkali metals and halogens.
(d) Loss of an electron from a hydrogen atom results in a nucleus of very small size as compared to other atoms or ions. Due to its small size it can not exist freely.
Ans: (iv) Loss of an electron from a hydrogen atom results in a nucleus of very small size as compared to other atoms or ions. Due to its small size it cannot exist free.
Loss of the electron from hydrogen atom results in nucleus size (H+) of 1.5 × 10-3. This is extremely small as compared to normal atomic and ionic sizes of 50 to 200 pm. As a consequence, H+ does not exist freely and is always associated with other atoms or molecules.
3. Metal hydrides are ionic, covalent or molecular in nature. Among LiH, NaH, KH, RbH, CsH, the correct order of increasing ionic character is
(a) LiH > NaH > CsH > KH > RbH
(b) LiH < NaH < KH < RbH < CsH
(c) RbH > CsH > NaH > KH > LiH
(d) NaH > CsH > RbH > LiH > KH
Ans: (ii) LiH < NaH < KH < RbH < CsH
In ionic hydride as it is formed by the s-Block element, down the group, electropositive character increases.
4. Which of the following hydrides is electron-precise hydride?
(a) B2H6
(b) NH3
(c) H2O
(d) CH4
Ans: (d) CH4
These hydrides have the required number of electrons to write their conventional Lewis structures.
5. Radioactive elements emit a, p and y rays and are characterized by their half lives. The radioactive isotope of hydrogen is
(a) Protium
(b) Deuterium
(c) Tritium
(d) Hydronium
Ans: (c) Tritium
The tritium concentration is about one atom per 1018 atoms of protium. Of these isotopes, only tritium is radioactive and emits low energy beta particles.
6. Consider the reactions
(A) H2O2 + 2HI → I2 + 2H2O
(B) HOCl + H2O2 → H3O+ + Cl- + O2
Which of the following statements is correct about H2O2 with reference to these reactions? Hydrogen peroxide is _______
(i) an oxidizing agent in both (A) and (B)
(ii) an oxidizing agent in (A) and reducing agent in (B)
(iii) a reducing agent in (A) and oxidizing agent in (B)
(iv) a reducing agent in both (A) and (B)
Ans: (ii)an oxidizing agent in (A) and reducing agent in (B)
H2O2 acts as an oxidising agent in A as it oxidized iodine from -1 to 0, while in B it acts as reducing agent as it reduced chlorine from +1 to -1
7. The oxide that gives H2O2 on treatment with dilute H2SO4 is
(i) PbO2
(ii) BaO2-8H2O
(iii) MnO2
(iv) TiO2
Ans: (ii) BaO2 -8H2O
Hydrogen peroxide is produced by acidifying barium peroxide and eliminating excess water by evaporation under low pressure.
8. Which of the following equations depicts the oxidizing nature of H2O2?
(i) 2MnO4- + 6H+ + 5H2O2 → 2Mn2+ + 8H2O + 5O2
(ii) 2Fe3+ + 2H+ + H2O2 → 2Fe2+ + 2H2O + O2
(iii) 2I- + 2H+ + H2O2 → I2+ 2H2O
(iv) KIO4 + H2O2→ KIO3 + H2O + O2
Ans:(iii) 2I- + 2H+ + H2O2 → I2+ 2H2O
As the oxidation state of iodine changes from -1 to 0, this shows peroxide is oxidising in nature.
9. Which of the following equations depicts the reducing nature of H2O2?
(i) 2[Fe(CN)6]4- + 2H+ + H2O2 → 2[Fe(CN)6]3- + 2H2O
(ii) I2 + H2O2 + 2OH–→ 2I-+ 2H2O + O2
(iii) Mn2+ + H2O2 → Mn4+ + 2OH
(iv) PbS + 4H2O2→ PbSO4 + 4H2O
Ans: (ii) I2 + H2O2 + 2OH–→ 2I-+ 2H2O + O2
As iodine is reduced from 0 to -1.
10. Hydrogen peroxide is _________.
(i) an oxidizing agent
(ii) a reducing agent
(iii) both an oxidizing and a reducing agent
(iv) neither oxidizing nor reducing agent.
Ans: (iii) both an oxidizing and a reducing agent
In both acidic and alkaline medium hydrogen peroxide can act as oxidizing and reducing agent.
11. Which of the following reactions increases production of Dihydrogen from synthesis gas?
(i) CH4(g)+H2O(g) → CO(g)+3H2(g)
(ii) C(g)+H2O(g) → CO(g)+H2(g)
(iii) CO(g)+H2O(g) → CO2(g)+H2(g)
(iv) C2H6+2H2O → 2CO+5H2O
Ans: (iii) CO(g)+H2O(g) → CO2(g)+H2(g)
The production of dihydrogen can be increased by reacting carbon monoxide of syngas mixtures with steam in the presence of iron chromate as catalyst.
12. When sodium peroxide is treated with dilute sulphuric acid, we get
(i) sodium sulphate and water
(ii) sodium sulphate and oxygen
(iii) sodium sulphate, hydrogen and oxygen
(iv) sodium sulphate and hydrogen peroxide.
Ans:(iv) sodium sulphate and hydrogen peroxide.
Na2O2+dil H2SO4 → Na2SO4 → Na2SO4+H2O2
13. Hydrogen peroxide is obtained by the electrolysis of _______.
(i) water
(ii) sulphuric acid
(iii) hydrochloric acid
(iv) fused sodium peroxide
Ans:(ii) sulphuric acid
2HSO4- (aq) → HO3SOOSO3H (aq) → 2HSO4- (aq)+2H+ (aq) + H2O2 (aq)
14. Which of the following reactions is an example of use of water gas in the synthesis of other compounds?
(i) CH4(g) + H2O(g) → CO(g) + H2(g)
(ii) CO(g) + H2O(g) → CO2(g) + H2(g)
(iii) CnH2n+2 + nH2O(g) → nCO + (2n+1)H2
(iv) CO(g)+2H2(g) → CH3OH(l)
Ans: (iv) CO(g)+2H2(g) → CH3OH(l)
The reaction shown in equation(iv) shows the synthesis of methanol from water gas.
15. Which of the following ions will cause hardness in the water sample?
(i) Ca2+
(ii) Na+
(iii) Cl-
(iv) K+
Ans: (i) Ca2+
Water becomes 'hard' when it contains calcium and magnesium salts in the form of hydrogen carbonate, chloride, and sulphate.
16. Which of the following compounds is used for water softening?
(i)Ca3(PO4)2
(ii) Na3PO4
(iii) Na6P6O18
(iv) Na2HPO4
Ans: (iii) Na6P6O18
Sodium hexametaphosphate(Na6P6O18), commercially called ‘calgon’, is used to remove hardness from water.
17. Elements of which of the following group(s) of periodic table do not form hydrides?
(i) Groups 7, 8, 9
(ii) Group 13
(iii) Groups 15, 16, 17
(iv) Group 14
Ans: (i) Groups 7,8, 9
Explanation: The metals belonging to group 7, 8 and 9 do not have tendency to form hydrides.
18. Only one element forms hydride.
(i) group 6
(ii) group 7
(iii) group 8
(iv) group 9
Ans: (i) group 6
From group 6, only chromium forms CrH.
MULTIPLE CHOICE QUESTIONS (TYPE-II)
In the following questions two or more options may be correct.
19. Which of the following statements are not true for hydrogen?
(i) It exists as a diatomic molecule.
(ii) It has one electron in the outermost shell.
(iii) It can lose an electron to form a cation which can freely exist.
(iv) It forms a large number of ionic compounds by losing an electron.
Ans: (iii) and (iv)
H+ does not exist freely and is always associated with other atoms or molecules. It has an extremely high ionisation enthalpy and does not have metallic properties under normal conditions, unlike alkali metals.
20. Dihydrogen can be prepared on a commercial scale by different methods. In its preparation by the action of steam on hydrocarbons, a mixture of CO and H2gas is formed. It is known as
(i) Water gas
(ii) Syngas
(iii) Producer gas
(iv) Industrial gas
Ans: (i) and (ii)
The combination of CO and H2 is known as water gas. This mixture of CO and H2 is also known as synthesis gas or ‘syngas' since it is used to make methanol and other hydrocarbons.
21. Which of the following statement(s) is/are correct in the case of heavy water?
(i) Heavy water is used as a moderator in nuclear reactor.
(ii) Heavy water is more effective as solvent than ordinary water.
(iii) Heavy water is more associated than ordinary water.
(iv) Heavy water has lower boiling point than ordinary water.
Ans:(i) and (iii)
Heavy water is utilised as a moderator in nuclear reactors and exchange reactions, and it is more associated with water due to its higher mass.
22. Which of the following statements about hydrogen are correct?
(i) Hydrogen has three isotopes of which protium is the most common.
(ii) Hydrogen never acts as cation in ionic salts.
(iii) Hydrogen ion, H+, exists freely in solution.
(iv) Dihydrogen does not act as a reducing agent.
Ans: (i) and (ii)
When an electron is removed from a hydrogen atom, the nucleus decreases to 1.5 × 10-3 pm in size. When compared to usual atomic and ionic sizes of 50 to 200 pm, this is exceedingly small. As a result, H+ does not exist independently and is always bound to other atoms or molecules.
23. Some of the properties of water are described below. Which of them is/ are not correct?
(i) Water is known to be a universal solvent.
(ii) Hydrogen bonding is present to a large extent in liquid water.
(iii) There is no hydrogen bonding in the frozen state of water.
(iv) Frozen water is heavier than liquid water.
Ans: (iii) and (iv)
The presence of extensive hydrogen bonding between water molecules gives it remarkable features in the condensed phase (liquid and solid phases). In comparison to H2S and H2Se, this results in a high freezing point, high boiling point, high heat of vaporisation, and high heat of fusion.
24. Hardness of water may be temporary or permanent. Permanent hardness is due to the presence of
(i) Chlorides of Ca and Mg in water
(ii) Sulphates of Ca and Mg in water
(iii) Hydrogen carbonates of Ca and Mg in water
(iv) Carbonates of alkali metals in water
Ans:(i) and (ii)
Presence of calcium and magnesium salts in the form of hydrogen carbonate, chloride and sulphate in water makes water ‘hard’.
25. Which of the following statements is correct?
(i) Elements of group 15 form electron deficient hydrides.
(ii) All elements of group 14 form electron precise hydrides.
(iii) Electron precise hydrides have tetrahedral geometries.
(iv) Electron rich hydrides can act as Lewis acids.
Ans: (ii) and (iii)
Electron precise hydrides have the required number of electrons to write their conventional Lewis structures. All elements of group 14 form such compounds (e.g., CH4) which are tetrahedral in geometry.
26. Which of the following statements is correct?
(i) Hydrides of group 13 act as Lewis acids.
(ii) Hydrides of group 14 are electron deficient hydrides.
(iii) Hydrides of group 14 act as Lewis acids.
(iv) Hydrides of group 15 act as Lewis bases.
Ans: (i) and (iv)
All elements in group 13 are Lewis acids because they form electron-deficient compounds. Excess electrons are found as lone pairs in electron-rich hydrides.
These compounds are made up of elements from groups 15-17. (NH3 has one lone pair, H2O has two, and HF has three.) As a result, they act as Lewis bases.
27. Which of the following statements is correct?
(i) Metallic hydrides are deficient in hydrogen.
(ii) Metallic hydrides conduct heat and electricity.
(iii) Ionic hydrides do not conduct electricity in solid state.
(iv) Ionic hydrides are very good conductors of electricity in solid state.
Ans: (i), (ii) and (iii)
Ionic hydrides are crystalline, non-volatile and non-conducting in solid state.
28. How can production of hydrogen from water gas be increased by using a water gas shift reaction?
Ans:Water-gas shift reaction: The water-gas shift reaction is a chemical reaction in which carbon monoxide reacts with steam in the presence of a catalyst to form carbon dioxide and hydrogen.
CO+H2+H2O → CO2+2H2
29. What are metallic/interstitial hydrides? How do they differ from molecular hydrides?
Ans: These are formed by many d-block and/block elements. However, the metals of group 7, 8 and 9 do not form hydride. Unlike saline hydrides, they are almost always non- stoichiometric, being deficient in hydrogen. For example, LaH 2.87, YbH 2.55, TiH 1.5-1.8, ZrH 1.3-1.75. Dihydrogen forms molecular compounds with most of the jp-block elements.
Most familiar examples are CH4, NH3, H2O and HF.
30. Name the classes of hydrides to which H2O, B2H6 and NaH belong.
Ans: NaH belongs to Saline hydrides and B2H6 and H2O belong to molecular hydrides.
31. If the same mass of liquid water and a piece of ice is taken, then why is the density of ice less than that of liquid water?
Ans: Ice has a highly ordered 3D hydrogen bonded structure. Each oxygen atom is surrounded tetrahedrally by four other oxygen atoms at a distance of 276 pm.
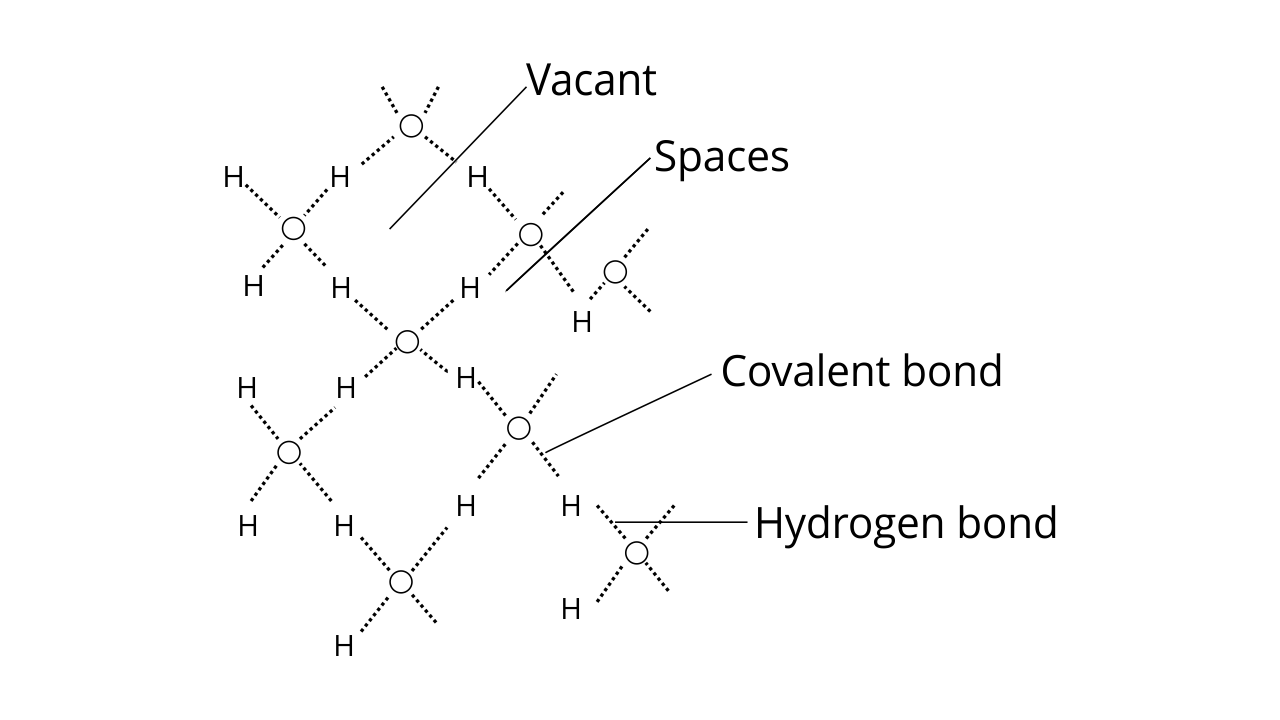
32. Complete the following equations:
i. PBS(s)+H2O2(aq) →
Ans: PBS(s)+4H2O2(aq) → PbSO4(s)+4H2O(l)
ii. CO(g)+H2(g) →
Ans: CO(g)+2H2(g) → CH3OH(l)
33. Give reasons:
(i) Lakes freeze from top towards bottom.
Ans: During winter, the temperature of lake water keeps on decreasing. Since cold water is heavier, it moves towards the bottom.
(ii) Ice floats on water.
Ans: Density of ice is less than that of water that is why it floats on the surface of water.
34. What do you understand about the term ‘autoprotolysis of water’? What is its significance?
Ans: Self ionization of water is known as autoprotolysis of water.
H2O(l) + H2O(l) → H2O2(aq) + OH-(aq)
Acid Base Conjugate acid Conjugate acid
Significance of autoprotolysis: Due to autoprotolysis, water has the ability to act as an acid as well as a base. Thus, it behaves as an amphoteric substance.
H2O(l)+NH3(aq) ↔ OH-(aq)+NH4+(aq)
H2O(l)+H2(aq) ↔ H3O+(aq)+HS-(aq)
35.Discuss briefly de-mineralisation of water by ion exchange resin.
Ans: Water demineralization using organic or synthetic ion exchange resins (ion exchange resins):
Synthetic resins are insoluble polymeric solids that contain a large hydrocarbon network with reactive acidic or basic groups.
These are better than Zeolite since they can remove all kinds of cations and anions from water. Demineralised or deionised water is the product of this process.
There are two categories of these:
Resins for cation exchange: Acidic groups such as COOH or SO3H can be found in them. Resin-H+-can be used to represent them.
Mg2+ + 2H-resin → Mg(resin)2+ 2H+
In hard water Cation exchanger
Ca2+ + 2H-resin →Ca(resin)2+2H+
In hard water cation exchanger
Anion exchange resins: They have basic groups such as OH- or NH2.
They may be represented as resin—OH- or resin.
SO42- + 2OH-resin → SO4-(resin)2+ 2OH-
Hard water anion exchanger
Cl- + OH-resin → Cl- resin +OH
Hard water anion exchanger
36. Molecular hydrides are classified as electron deficient, electron precise and electron rich compounds. Explain each type with two examples.
Ans: (a)Electron deficient hydrides are those that lack sufficient number of electrons to form typical covalent bonds. Group 13 hydrides, for example (BH3, AlH3, etc.).
(b) Electron precise: Electron precise hydrides are those that have the exact number of electrons required to form covalent bonds. Group 14 hydrides, for example (CH4, SiH4, GeH4, SnH4, PbH4 etc.). They are tetrahedral in shape.
(c) Electron rich hydrides: Electron rich hydrides are those that have more electrons than are required to create conventional covalent bonds. Hydrides of groups 15 to 17. (NH3, PH3, H2O, H2S, H2Se, H2Te, HF etc.).
37. How is heavy water prepared? Compare its physical properties with those of ordinary water.
Ans: Heavy water can be made by electrolyzing water repeatedly or as a by-product in the fertiliser industry. It's a deuterium compound that's used to make other deuterium compounds.
Physical Properties of H2O and D2O
Property | H2O | D2O |
Molecular mass (g mol-1) | 18.015 | 20.0276 |
Melting point/K | 273.0 | 276.8 |
Boiling point/K | 373.0 | 374.4 |
Enthalpy of formation/kJ mol- | -285.9 | -294.6 |
Enthalpy of Vaporisation (373 K)/kJ mol-1 | 40.66 | 41.61 |
Enthalpy of fusion/kJ mol-1 | 6.01 | — |
Temp of max. density/K | 276.98 | 284.2 |
Density (298 K)/g cm-3 | 1.0000 | 1.1059 |
Viscosity/centipoise | 0.8903 | 1.107 |
Dielectric constant/C2/N.m2 | 78.39 | 78.06 |
38.Write one chemical reaction for the preparation of D2O2.
Ans: Peroxodisulphate, obtained by electrolytic oxidation of acidified sulphate solutions at high current density, on hydrolysis yields hydrogen peroxide.
2HSO4-(aq) → HO3SOOSO3 \[\xrightarrow[\Delta ]{Hydrolysis}\] 2HSO4-(aq)+2H+(aq)+H2O2(aq)
This method is now used for the laboratory preparation Of D2O2.
K2S2O8(s)+2D2O(l) → 2KDSO4(aq)+D2O2(l)
39. Calculate the strength of the 5 volume H2O2 solution.
Ans: 2H2O2 → 2H2O+O2
5 volume H2O2 means IL of H2O2 will give 5L of O2 at STP.
On the basis of the equation it is clear that 22.7 L of O2 is produced by 68 g of H2O2.
5L of O2 is produced by = \[\frac{\text{68g }\!\!\times\!\!\text{ 51}}{\text{22}\text{.71}}\,\text{=}\,\frac{\text{3400}}{\text{227}}\] g of H2O2=14.9g
i.e. 15 g H2O2 dissolved in 1 L solution will give 5 L oxygen or 1.5 g H2O2/100 mL solution will give 500 mL oxygen. Thus 15 g/L or 1.5% solution is known as the 5V solution of H2O2.
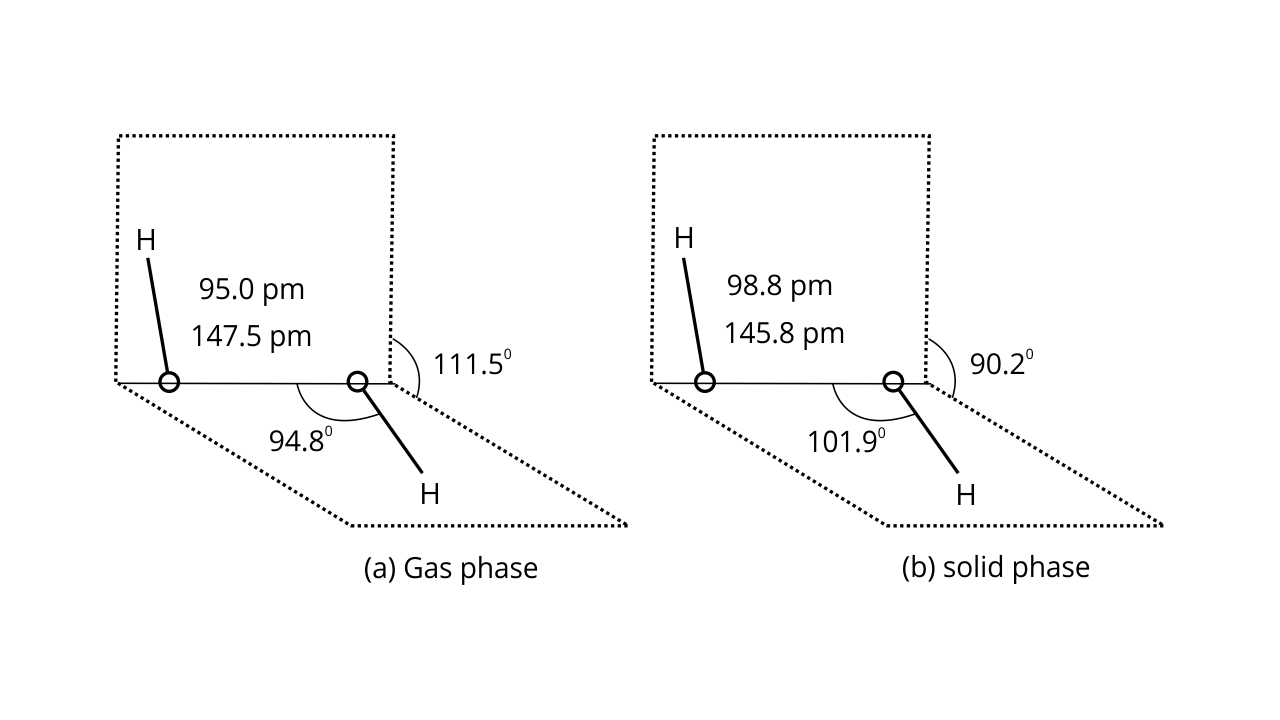
40. (i) Draw the gas phase and solid phase structure of H2O2.
Ans:
(ii) H2O2 is a better oxidising agent than water. Explain.
Ans: In H2O2 oxygen is in -1 oxidation state which has tendency to become -2 that is why it is a better oxidising agent than water.
41. Melting point, enthalpy of vaporization and viscosity data of H2O and D2O is given below:
On the basis of this data explain in which of these liquids intermolecular forces are stronger?
Ans: D2O has greater intermolecular force of attraction.
42. Dihydrogen reacts with dioxygen (O2) to form water. Write the name and formula of the product when the isotope of hydrogen which has one proton and one neutron in its nucleus is treated with oxygen. Will the reactivity of both the isotopes be the same towards oxygen? Justify your answer.
Ans: Dihydrogen reacts with dioxygen to form water. The reaction is highly exothermic.
H2(g) + O2(g) → H2O(l)
∆H = -285.9KJmol-1
The isotope of hydrogen which has one proton and one neutron is deuterium. When it reacts with O2 it forms Deuterium oxide (D2O). Deuterium reacts in a similar way but the reactivity will be lesser than hydrogen because of high bond dissociation enthalpy of D2 as compared to hydrogen.
43. Explain why HCl is a gas and HF is a liquid.
Ans: Because of hydrogen bonding HF is liquid whereas HCl is gas as there is lack of hydrogen bonding in HCl.
44. When the first element of the periodic table is treated with dioxygen, it gives a compound whose solid state floats on its liquid state. This compound has an ability to act as an acid as well as a base. What products will be formed when this compound undergoes auto ionization?
Ans:The compound is water which undergoes self ionization.
The autoprotolysis (self-ionization) of water takes place as follows:
H2O(l) + H2O(l) → H3O+(aq) + OH-(aq)
Acid Base Conjugate acid Conjugate acid
45. Rohan heard that instructions were given to the laboratory attendant to store a particular chemical i.e., keep it in the dark room, add some urea in it, and keep it away from dust. This chemical acts as an oxidising as well as a reducing agent in both acidic and alkaline media. This chemical is important for use in the pollution control treatment of domestic and industrial effluents.
(i) Write the name of this compound.
Ans: The given compound is H2O2.
(ii) Explain why such precautions are taken for storing this chemical.
Ans: Textiles, paper pulp, leather, oils, fats, and other products use it as a bleaching agent. In both acidic and alkaline media, it works as an oxidising and reducing agent. It is therefore kept in dark wax-lined glass or plastic containers. As a stabiliser, urea might be used. It is kept away from dust since dust can cause the compound to decompose explosively.
46. Give reasons why hydrogen resembles alkali metals?
Ans: Hydrogen has electronic configuration 1s1. On the one hand, its electronic configuration is similar to that of alkali metals in the first group of the periodic table, which have an outer electronic configuration (ns1). As a result, hydrogen resembles alkali metals that lose one electron to become unipositive ions.
47. Hydrogen generally forms covalent compounds. Give a reason.
Ans: Because of ionization enthalpy, hydrogen resembles more with halogens, is 520 kJ mol-1, F is 1680 kJ mol-1and that of His 1312 kJ mol-1. Like halogens, it forms a diatomic molecule, combining with elements to form hydrides and a large number of covalent compounds.
48.Why is the ionisation enthalpy of hydrogen higher than that of sodium?
Ans: In sodium the last shell electron is in 3s1 after losing that electron, it can acquire the configuration of Ne. Whereas in hydrogen, the electron is in s-orbital.
49. Basic principle of hydrogen economy is transportation and storage of energy in the form of liquid or gaseous hydrogen. Which property of hydrogen may be useful for this purpose? Support your answer with the chemical equation if required.
Ans: Hydrogen economy (Hydrogen as fuel):
(i) The electricity cannot be stored in automobiles. It is not possible to store and transport nuclear energy. Hydrogen is an alternative source of energy and hence called the ‘hydrogen economy". Hydrogen has some advantages as fuel.
(ii) Available in abundance in combined form as water.
(iii) On combustion produces H2O. Hence, pollution free.
(iv) H2O2 fuel cells give more power.
(v) Excellent reducing agent. Therefore, it can be used as a substitute of carbon in reduction for processes in industry.
50. What is the importance of heavy water?
Ans: Heavy water can be used as a moderator in nuclear reactors and in exchange reactions for the study of reaction mechanisms. It can be prepared by exhaustive electrolysis of water or as a by-product in some fertilizer industries.
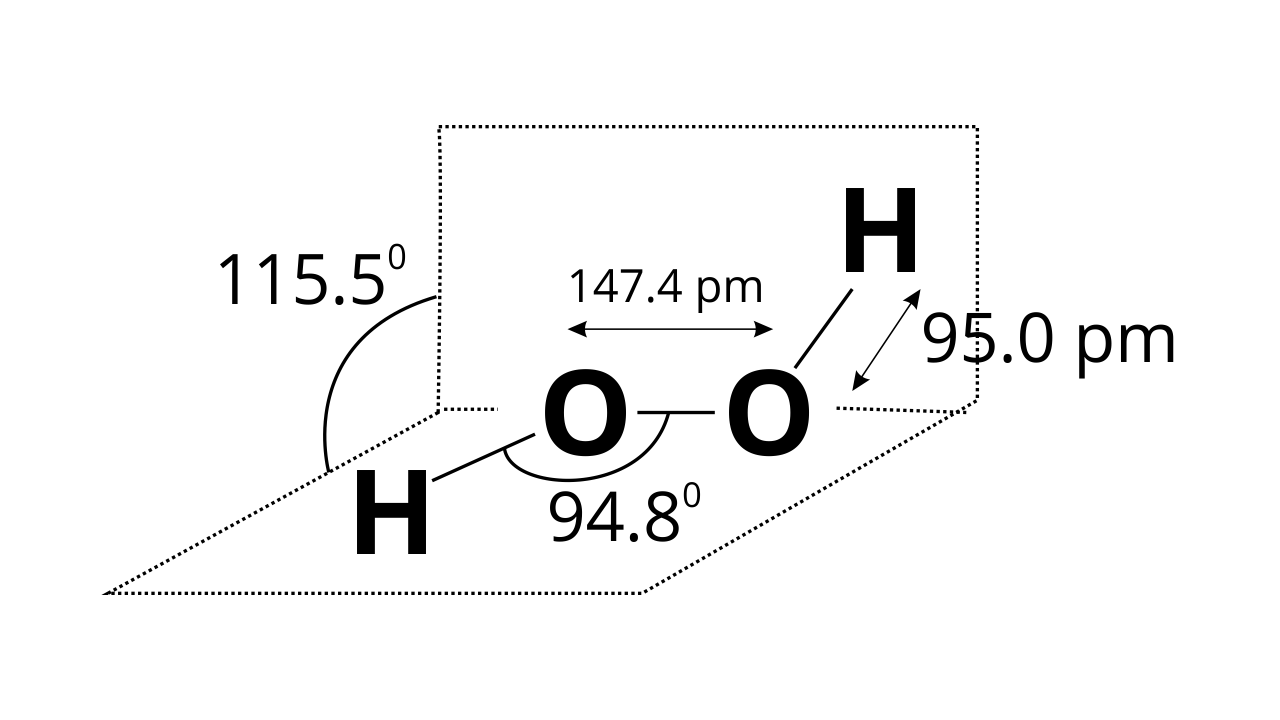
51. Write the Lewis structure of hydrogen peroxide.
Ans: H2O2 structure in gas phase, dihedral angle is 111.50. H2O2 structure in solid phase at 110 k, dihedral angle is 90.20.

52. An acidic solution of hydrogen peroxide behaves as an oxidising as well as reducing agent. Illustrate it with the help of a chemical equation.
Ans: (i) Oxidising action in acidic medium:
2Fe2+(aq)+2H+(aq)+H2O2(aq) → 2Fe3+(aq)+2H2O(l)
PbS(s)+4H2O2(aq) → PbSO4(s)+4H2O(l)
(ii) Reducing action in acidic medium:
2MnO4-+6H++5H2O2 → 2Mn2++8H2O+5O2
HOCl+H2O2 → H3O++Cl-+H2O
53.With the help of suitable examples, explain the property of H2O2 that is responsible for its bleaching action?
Ans:H2O2 decomposes slowly on exposure to light.
2H2O2→ 2H2O+2O2
In daily life it is used as a hair bleach and as a mild disinfectant. As an antiseptic it is sold in the market as perhydrol.
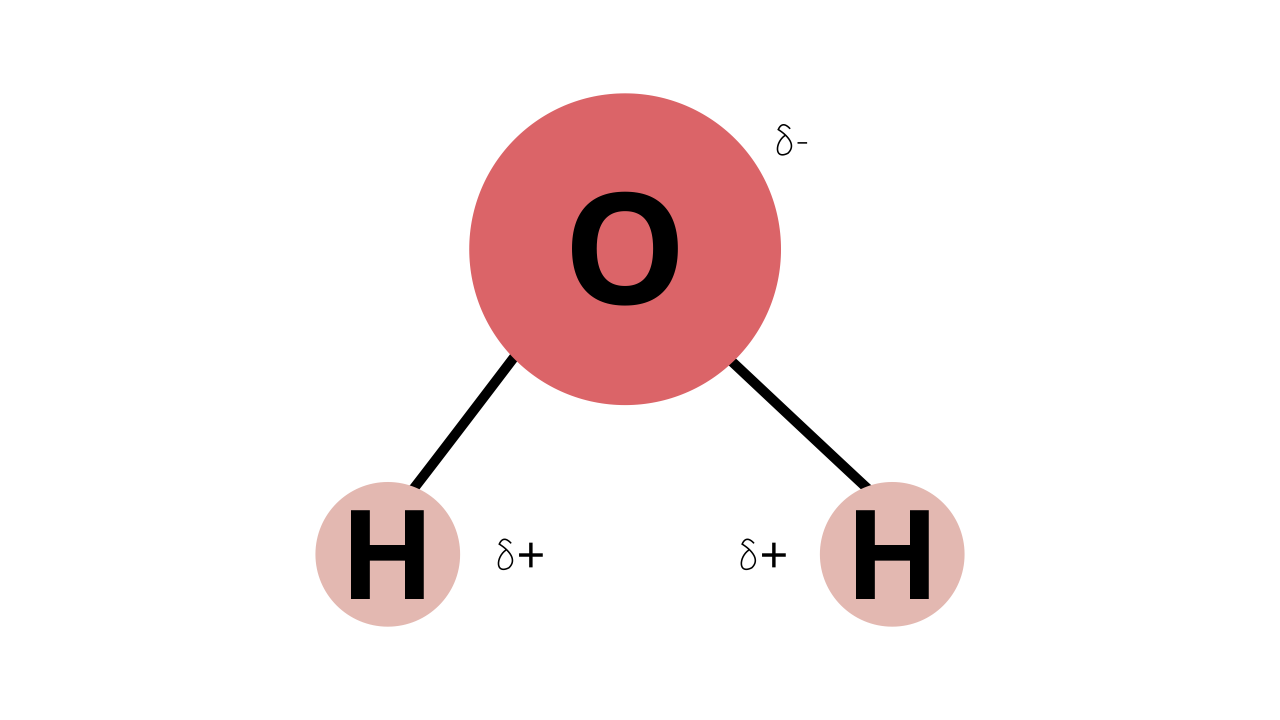
54.Why are water molecules polar?
Ans: Water molecules are polar because in the gas phase water is a bent molecule with a bond angle of 104.5°, O-H bond length of 95.7 pm.
55. Why does water show a high boiling point as compared to hydrogen sulphide? Give reasons for your answer.
Ans: Because of hydrogen bonding water has a higher boiling point than H2S as in H2S hydrogen bonding is absent.
56.Why can dilute solutions of hydrogen peroxide not be concentrated by heating? How can a concentrated solution of hydrogen peroxide be obtained?
Ans: Hydrogen peroxide cannot be concentrated by heating as it can cause bumps on heating. It can be extracted with water and concentrated to ~30% (by mass) by distillation under reduced pressure. It can be further concentrated to ~85% by careful distillation under low pressure. The remaining water can be frozen out to obtain pure H2O2.
57. Why is hydrogen peroxide stored in wax lined bottles?
Ans: Hydrogen peroxide is stored in wax lined bottles because decomposes slowly on exposure to light.
58. Why does hard water not form lather with soap?
Ans: With soap, hard water generates scum/precipitate. Soap containing sodium stearate (C117H35COONa) interacts with hard water to form calcium and magnesium stearate (Ca/Mg stearate). As a result, it is unsuitable for laundering. Because of the deposition of salts in the form of scale, it is also hazardous to boilers.
59. Phosphoric acid is preferred over sulphuric acid in preparing hydrogen peroxide from peroxides. Why?
Ans: Because H2SO4 can activate the decomposition of H2O2.
60. How will you account for the 104.5° bond angle in water?
Ans: In water hybridisation of oxygen is sp3. Angle should be109o(approx.) but due to LP - LP repulsion bond angle reduces to 104.5°.
61.Write redox reaction between fluorine and water.
Ans:2F2(g)+2H2O → 4H+(aq)+4F-(aq)+O2(g)
62. Write two reactions to explain the amphoteric nature of water.
Ans:H2O(l)+NH3(aq) → OH-(aq)+NH4+(aq)
H2O(l)+H2S(aq) → H3O+(aq)+HS-(aq)
63. Correlate the items listed in Column I with those listed in Column II. Find out as many correlations as you can.
Column I | Column II |
(i) Synthesis gas | a. Na2[Na4(PO3)6] |
(ii) Dihydrogen | (b) Oxidising agent |
(iii) Heavy water | (c) Softening of water |
(iv) Calgon | (d) Reducing agent |
(v) Hydrogen peroxide | (e) Stoichiometric compounds of s-block elements |
(vi) Salt like hydrides | (f) Prolonged electrolysis of water |
(g) Zn + NaOH | |
(h) Zn + dil. H2SO4 (i) Synthesis of methanol | |
(j) Mixture of CO and H2 |
Ans:(i) → (j); (ii) → (h); (iii) → (f) : (iv) → (a); (v) → (b); (vi) → (e)
64. Match Column I with Column II for the given properties/applications mentioned therein.
Column I | Column II |
(i) H | (a) Used in the name of perhydrol. |
(ii)H2 | (b) Can be reduced to dihydrogen by NaH. |
(iii)H2O | (c) Can be used in hydroformylation of olefin. |
(iv) H2O2 | (d) Can be used in cutting and welding. |
Ans:(i)→(d); (ii)→(b); (iii)→(c); (iv)→(a)
65. Match the terms in Column I with the relevant item in Column II.
Column I | Column II |
(i) Electrolysis of water produces | (a) atomic reactor |
(ii)Lithium aluminum hydride is used as | (b) polar molecule |
(iii)Hydrogen chloride is a | (c) recombines on metal surface to generate high temperature |
(iv) Heavy water is used in | (d) reducing agent |
(v) Atomic hydrogen | (e) hydrogen and oxygen |
Ans:(i)→(e); (ii)→(d); (iii)→(b); (iv)→(a); (v)→(c)
66. Match the items in Column I with the relevant item in Column II.
Column I | Column II |
(i) Hydrogen peroxide is used as a | (a) zeolite |
(ii)Used in Calgon method | (b) perhydrol |
(iii)Permanent hardness of hard water is removed by | (c) sodium hexametaphosphate |
(d) propellant |
Ans:(i) → (b); (ii) → (c); (iii) → (a)
Assertion and reason type
In the following questions a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct option out of the choices given below each question.
36. Assertion (A): Permanent hardness of water is removed by treatment with washing soda.
Reason (R): Washing soda reacts with soluble magnesium and calcium sulphate to form insoluble carbonates.
(i) Statements A and R both are correct and R is the correct explanation of A.
(ii) A is correct but R is not correct.
(iii) A and R both are correct but R is not the correct explanation of A.
(iv) A and R both are false.
Ans:(i)Statements A and R both are correct and R is the correct explanation of A.
MCl2+Na2CO3→ MCO3+2NaCl
MSO4+Na2CO3→MCO3+Na2SO4
68. Assertion (A): Some metals like platinum and palladium, can be used as storage media for hydrogen.
Reason (R): Platinum and palladium can absorb large volumes of hydrogen.
(i)Statements A and R both are correct and R is the correct explanation of A.
(ii)A is correct but R is not correct.
(iii)A and R both are correct but R is not the correct explanation of A.
(iv)A and R both are false.
Ans:(i) Statements A and R both are correct and R is the correct explanation of A. Since, metals like Pd and Pt adsorbs a large volume of hydrogen, hence, these are used as a storage media for it.
Long Answer Type
69. Atomic hydrogen combines with almost all elements but molecular hydrogen does not. Explain.
Ans: Atomic hydrogen is highly reactive, whereas molecular hydrogen is rather inert. Bond dissociation enthalpy determines the chemical behaviour of dihydrogen (and, for that matter, any molecule) to a great extent. For a single bond between two atoms of any element, the H-H bond dissociation enthalpy is the highest. As a result, molecular hydrogen reacts only with a few elements.
70. How can D2O be prepared from water? Mention the physical properties in which D2O differs from H2O. Give at least three reactions of D2O showing the exchange of hydrogen with deuterium.
Ans: D2O can be prepared by prolonged electrolysis of water. Due to high molecular mass D2O differs from water.
NaOH+D2O → NaOD+HOD
HCl+D2O → DCl+ HOD
NH4Cl+D2O → NH3DCl+HOD
Physical Properties of H2O and D2O
Property | H2O | D2O |
Molecular mass (g mol-1) | 18.015 | 20.0276 |
Melting point/K | 273.0 | 276.8 |
Boiling point/K | 373.0 | 374.4 |
Enthalpy of formation/kJ mol-1 | -285.9 | -294.6 |
Enthalpy of Vaporisation (373 K)/kJ mol-1 | 40.66 | 41.61 |
Enthalpy of fusion/kJ mol-1 | 6.01 | — |
Temp of max. density/K | 276.98 | 284.2 |
Density (298 K)/g cm-3 | 1.0000 | 1.1059 |
Viscosity/centipoise | 0.8903 | 1.107 |
Dielectric constant | 78.39 | 78.06 |
71. How will you concentrate H2O2? Show differences between structures of H2O2 and H2O by drawing their spatial structures. Also mention three important uses of H2O2.
Ans: Hydrogen peroxide is produced by acidifying barium peroxide and eliminating surplus water by evaporation under low pressure. Water is used to extract it, then distillation under lower pressure concentrates it to around 30% (by mass). Careful distillation under low pressure can increase the concentration to 85%. To achieve pure H2O2, the residual water can be frozen out.
Spatial structures of H2O2 and H2O:
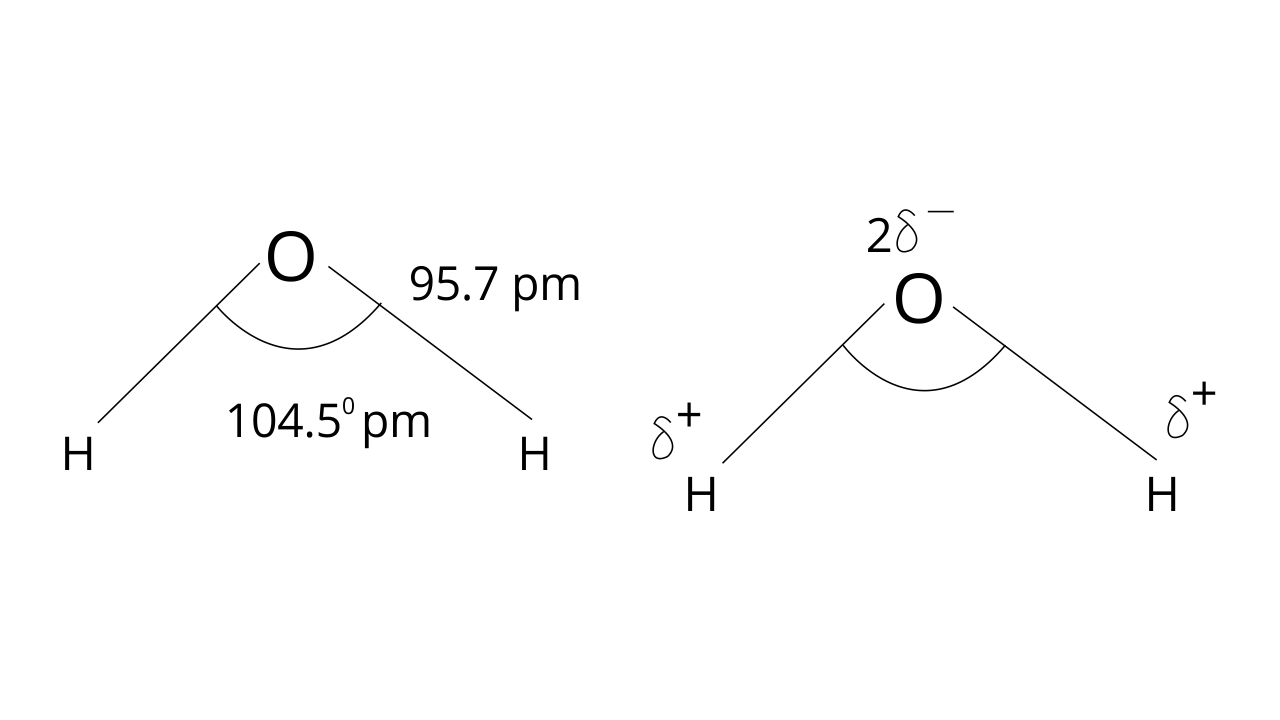

Uses of H2O2:
(i) As an antiseptic it is sold in the market as perhydrol.
(ii) It is used to manufacture chemicals like sodium perborate and per- carbonate. It is employed in the industries as a bleaching agent for textiles.
(iii) It is used in laboratories as an oxidising agent. Also used in rocket fuels.
72. (i) Give a method for the manufacture of hydrogen peroxide and explain the reactions involved therein.
Ans: (i) Industrial preparation: H2O2 is prepared by the auto-oxidation of 2- alkylanthraquinols
2-ethylanthraquinol ↔ H2O2+ oxidised product
2Fe2+(aq)+2H+(aq)+H2O2 → Fe3(aq)+2H2O(l)
PbS(s)+4H2O2(l) → PbSO4(s)+4H2O(l)
(ii) Illustrate oxidising, reducing and acidic properties of hydrogen peroxide with equations.
Ans: Reducing action of hydrogen peroxide
2MnO4-+6H++5H2O2→ 2Mn2++8H2O+5H2
HOCl+H2O2 → H3O++Cl-+O2
Oxidising action of hydrogen peroxide
2Fe2++H2O2→ 2Fe3++2OH-
Mn2++H2O2 → Mn4++2OH-
Acidic properties of H2O2
I2+H2O2+2OH-→ 2I-+2H2O+H2
2MnO4-+3H2O2→ 2MnO2+3O2+2H2O+2OH-
73. What mass of hydrogen peroxide will be present in 2 litres of a 5 molar solution? Calculate the mass of oxygen which will be liberated by the decomposition of 200 mL of this solution.
Ans: Mass of H2O2 = 68 g.
Mol. Mass of H2O2=34gmol-1
∴1L of M solution of H2O2 will contain H2O2=34×5 g
∴2L of 5 M solution will contain H2O2=34×5×2=340g
or 200 mL of 5 solution will contain H2O2 = \[\frac{\text{340}}{\text{2000}}\] × 200 = 34 g
Now 2H2O2 → 2H2O+O2
64 g 32g
Now 68 g of H2O2 on decomposition will give O2=32g
∴34g of H2O2 on decomposition will give O2 = \[\frac{\text{32}}{68}\] × 34=16 g
74.A colourless liquid ‘A’ contains H and O elements only. It decomposes slowly on exposure to light. It is stabilised by mixing urea to store in the presence of light.
(i)Suggest possible structure of A.
Ans: Since, a colourless liquid 'A' contains only hydrogen and oxygen and decomposes slowly on exposure to light but is stabilised by addition of urea, therefore, liquid A may be hydrogen peroxide. A is H2O2.
Structure of H2O2 is given below.
(ii)Write chemical equations for its decomposition reaction in light.
Ans: Reaction for decomposition of H2O2 in presence of light:
2H2O2(l) → 2H2O(l)+O2(g)
75. An ionic hydride of an alkali metal has significant covalent character and is almost unreactive towards oxygen and chlorine. This is used in the synthesis of other useful hydrides. Write the formula of this hydride. Write its reaction with Al2Cl6.
Ans: It is lithium hydride (LiH) because it has significant covalent character due to the smallest alkali metal, LiH is very stable. It is almost nonreactive towards oxygen and chlorine. It reacts with Al2Cl2 to form lithium aluminium hydride.
8LiH+ Al2Cl6 → 2LiAlH4 → LiCl
76. Sodium forms a crystalline ionic solid with Dihydrogen. The solid is non-volatile and non-conducting in nature. It reacts violently with water to produce Dihydrogen gas. Write the formula of this compound and its reaction with water. What will happen on electrolysis of the melt of this solid?
Ans: Sodium reacts with Dihydrogen to form sodium hydride which is a crystalline ionic solid.
2Na+H2 → 2Na+H-
It reacts with H2O to produce H2 gas
2NaH+2H2O → 2NaOH+2H2
Although Na+H- does not conduct electricity in the solid state, the electrolysis of its melt produces H2 at the anode and Na at the cathode.
At cathode At anode
Na+H-(l) \[\xrightarrow{Electrolysis}\] 2Na(l) + H2(g)
Their melts conduct electricity and on electrolysis liberate Dihydrogen gas at anode, which confirms the existence of H-ion.
The Physical Properties of Hydrogen are-
Hydrogen is a gas with no color and no odor
Hydrogen has the least density of all gases.
Hydrogen is viewed as the clean fuel of the future which is developed from water and returned back to water when oxidized.
Hydrogen is present in almost all molecules in living things.
It remains connected with carbon and oxygen atoms.
Hydrogen is the most plentiful element in the whole universe. It is present as a gas in the atmosphere in 1 part per million volume.
It is spotless, non-toxic, and safe to produce from various sources.
It is named an energy carrier because it stores energy that is first created somewhere else.
FAQs on NCERT Exemplar for Class 11 Chemistry Chapter-9 (Book Solutions)
1. What are the uses of Deuterium and Tritium?
Deuterium consists of one proton and one neutron in its nucleus. Deuterium is not radioactive and is used in chemical analysis and for preparing solvents for Hydrogen. Some of the other uses of Deuterium are-
Preparing Essential Drugs
Building Nuclear weapons
Preparing prototype fusion reactors
Tracing
NMR spectroscopy
Tritium comprises two neutrons and one proton in its nucleus. Some of the uses of Tritium are-
Analytical Chemistry
Used to control nuclear fusion
Tritium in Hydrogen bomb secondaries
Neutron initiator
Preparation of Nuclear weapons
Self-powered lighting
Oceanic transient tracer
2. What are Metallic Hydrides?
Metal Hydride is the Hydrogen compound that forms a bond with other metal elements. Mostly, The bond is covalent type, but sometimes the Hydrides are formed with the ionic bonds. Metal Hydrides are usually formed by transition metals and are mostly hard, have high melting & boiling points, and are non-stoichiometric. For Example - Cadmium, Magnesium, etc. Metal Hydrides are also called Interstitial Hydrides. These are formed when the Hydrogen molecule reacts with the d-block or f-block elements. The metals of chemical groups 7, 8, and 9 can never form Hydrides. They do conduct electricity and heat but not to the extent of other metals.
3. Distinguish between Hydration and Hydrolysis.
The major difference between Hydrolysis and Hydration is that Hydrolysis is called the process of breaking compounds using water, whereas Hydration is called the electrophilic addition reaction with no cleavage of the original molecule. In other words, the process of Hydration leaves the non-water components intact. Hydrolysis is the breakdown procedure of molecules while it reacts with water. This is the major difference between Hydrolysis and Hydration. Class 12 students can learn more about Hydration and Hydrolysis at Vedantu's website.
4. What are the uses of Hydrides?
The compounds of Hydrogen with low electronegative elements are called Hydrides. Whenever Hydrogen reacts with any element, the product formed is considered to be a Hydride. Some uses of Hydrides are-
Hybrids are used as reducing agents in many chemical industries.
Hydrides are highly significant in battery storage technologies. For Example- Nickel Hydride Batteries.
Hydrides have been used as drying agents for a long time
Hydrides are also used as strong bases in organic synthesis.
Metal Hydrides are used for their Hydrogen storage, compressors' capabilities, and heat storage.
5. What are the categories of Hydrides?
The compounds of Hydrogen with low electronegative elements are known as Hydrides. Hydrides are divided into 3 parts-
Ionic Hydrides- Ionic Hydrides are constructed when a Hydrogen molecule reacts with highly electropositive s-block elements. In solid-state, the ionic Hydrides are crystalline, non-conducting, and non-volatile. Ionic Hydrides conduct electricity in a liquid state. Ionic Hydrides on electrolysis liberate Hydrogen gas at the anode. These are also called Saline Hydrides.
Covalent Hydrides- Covalent Hydrides are constructed when the Hydrogen reacts with other identical electronegative elements like Silicon, Cabon, etc. Covalent Hydrides are compounds that are formed when Hydrogen is reacted with non-metals. The compound transmits a covalent bond and is either a volatile or non-volatile compound. Covalent Hydrides can also be liquids or gases.
Metallic Hydrides- Metal Hydrides are Hydrogen compounds that form a bond with another metal element. The bond is a mostly covalent type but occasionally the Hydrides are constructed with ionic bonds. These are usually formed by transition metals and are mostly hard in nature, non-stoichiometric, high melting, and boiling points.

























