NCERT Exemplar for Class 11 Chemistry - The S-Block Elements - Free PDF Download
Chapter 10 of Chemistry, the s-Block Elements, revolves around the two groups of periodic table groups 1 and 2 named Alkali metals and Alkaline earth metals respectively, these two groups are the part of s-block elements, which contains a total of 13 elements. Students can download free PDFs of all the NCERT exemplar questions in this chapter from the official website of Vedantu.
Free PDF download of NCERT Exemplar for Class 11 Chemistry Chapter 10 - The s-Block Elements solved by expert Chemistry teachers on Vedantu as per NCERT (CBSE) Book guidelines. All Chapter 10 - The s-Block Elements exercise questions with solutions to help you to revise the complete syllabus and score more marks in your examinations.
Access NCERT Exemplar Solutions for Class 11 Chemistry Chapter 10 - The S-Block Elements
I. Multiple Choice Questions (Type-I)
1. The alkali metals are low melting. Which of the following alkali metal is expected to melt if the room temperature rises to 30°C?
(A) Na
(B) K
(C) Rb
(D) Cs
Ans: Correct Option(D)
Explanation:
In alkali metals, on going down the group, as the metallic strength decreases, melting point decreases. Thus, Cs has the lowest melting point and melts at 30°C.
2. Alkali metals react with water vigorously to form hydroxides and dihydrogen. Which of the following alkali metals reacts with water least vigorously?
(A) $\mathrm{Li}$
(B) $\mathrm{Na}$
(C) $\mathrm{K}$
(D) $\mathrm{CS}$
Ans: Correct Option(A)
Explanation:
The reactivity of alkali metals increases on moving down the group. So, $\mathrm{Li}$ is least reactive. It will react with water least vigorously. So, option (A) is correct.
3. The reducing power of a metal depends on various factors. Suggest the factor which makes Li, the strongest reducing agent in aqueous solution.
(A) Sublimation enthalpy
(B) lonisation enthalpy
(C) Hydration enthalpy
(D) Electron-gain enthalpy
Ans: Correct Option(C)
Explanation:
Li due to its small size it has a high value of hydration enthalpy. Due to the high hydration enthalpy of $\mathrm{Li}$ atom, it is the strongest reducing agent in the aqueous medium.
So, option (C) is correct.
4. Metal carbonates decompose on heating to give metal oxide and carbon dioxide. Which of the metal carbonates is most stable thermally?
(A) $\mathrm{MgCO}_{3}$
(B) $\mathrm{CaCO}_{3}$
(C) $\mathrm{SrCO}_{3}$
(D) $\mathrm{BaCO}_{3}$
Ans: Correct option(D)
Explanation:
$\mathrm{BaCO}_{3}$ will be the most thermal stable. The size of $\mathrm{Ba}^{2+}$ is very large due to which it has low Polarising power and cannot polarise the oxygen atom. As the positive ions gets larger on moving down the group, the effect on the carbonate ion near them decreases. Thus, the carbonate ions of large cations are stable.
So, option (D) is correct.
5. Which of the carbonates given below is unstable in air and is kept in $\mathrm{CO}_{2}$ atmosphere to avoid decomposition.
(A) $\mathrm{BeCO}_{3}$
(B) $\mathrm{MgCO}_{3}$
(C) $\mathrm{CaCO}_{3}$
(D) $\mathrm{BaCO}_{3}$
Ans: Correct option(A)
Explanation:
Due to the smaller size of the cation $\mathrm{Be}^{2+}$, and larger size of the anion $\mathrm{CO}_{3}^{2-}, \mathrm{BeCO}_{3}$ is unstable in air. Therefore, it is kept in $\mathrm{CO}_{2}$ atmosphere to avoid decomposition.
So, option (A) is correct.
6. Metals form basic hydroxides. Which of the following metal hydroxide is the least basic?
(A) $\mathrm{Mg}(\mathrm{OH})_{2}$
(B) $\mathrm{Ca}(\mathrm{OH})_{2}$
(c) $\mathrm{Sr}(\mathrm{OH})_{2}$
(D) $\mathrm{Ba}(\mathrm{OH})_{2}$
Ans: Correct option(A)
Explanation:
As the size of the metal increases, the basic character of the hydroxide increases down the group. The solubility of hydroxide increases, and hydroxide of Be and $\mathrm{Mg}$ is almost insoluble. So, option (A) is correct.
7. Some of the Group 2 metal halides are covalent and soluble in organic solvents. Among the following metal halides, the one which is soluble in ethanol is
(A) $\mathrm{BeCl}_{2}$
(B) $\mathrm{MgCl}_{2}$
(C) $\mathrm{CaCl}_{2}$
(D) $\mathrm{SrCl}_{2}$
Ans: Correct option(A)
Explanation:
$\mathrm{BeCl}_{2}$ is covalent in nature due to the high polarising power of beryllium(+2) ion. On moving down the group, as the size increases, covalent nature decreases. Therefore, beryllium chloride being most covalent is soluble in ethanol.
8. The order of decreasing jojisation enthalpy in alkali metals is
(A) $\mathrm{Na}>\mathrm{Li}>\mathrm{K}>\mathrm{Rb}$
(B) $\mathrm{Rb}<\mathrm{Na}<\mathrm{K}<\mathrm{Li}$
(C) $\mathrm{Li}>\mathrm{Na}>\mathrm{K}>\mathrm{Rb}$
(D) $\mathrm{K}<\mathrm{Li}<\mathrm{Na}<\mathrm{Rb}$
Ans: Correct Option(C)
Explanation:
On moving down the group as the size increases, the nuclear charge attraction decreases. The effect of increasing size outweighs the increasing nuclear charge. Thus, ionization enthalpy decreases. The order of decreasing ionization enthalpy is,
$\mathrm{Li}>\mathrm{Na}>\mathrm{K}>\mathrm{Rb}$
9. The solubility of metal halides depends on their nature, lattice enthalpy and hydration enthalpy of the individual ions. Amongst fluorides of alkali metals, the lowest solubility of LiF in water is due to (A) lonic nature of lithium fluoride
(B) High lattice enthalpy
(C) High hydration enthalpy for lithium ion.
(D) Low ionisation enthalpy of lithium atom
Ans: Correct option(B)
Explanation:
The solubility of salt depends on the lattice and hydration energy both. By increasing lattice energy and decreasing hydration energy solubility decreases. In case of biE, both the ions are small, thus the compound has high lattice energy and is insoluble in water. If both the cations and anions are comparable in size, then stability is maximum and solubility is minimum.
10. Amphoteric hydroxides react with both alkalies and acids. Which of the following Group 2 metal hydroxides is soluble in sodium hydroxide?
(A) $\mathrm{Be}(\mathrm{OH})_{2}$
(B) $\mathrm{Mg}(\mathrm{OH})_{2}$
(C) $\mathrm{Ca}(\mathrm{OH})_{2}$
(D) $\mathrm{Ba}(\mathrm{OH})_{2}$
Ans: Correct option(A)
Explanation:
$\mathrm{Be}(\mathrm{OH})_{2}$ is amphoteric. It reacts with both acids and bases. It reacts with acid to form beryllium chloride and it reacts with base to form beryllate ion which is soluble in sodium hydroxide. The reaction is shown below.
$\mathrm{Be}(\mathrm{OH})_{2}+2 \mathrm{OH}^{-} \rightarrow\left[\mathrm{Be}(\mathrm{OH})_{4}\right]^{2-}$
$\mathrm{Be}(\mathrm{OH})_{2}+2 \mathrm{HCl} \rightarrow \mathrm{BeCl}_{2}+2 \mathrm{H}_{2} \mathrm{O}$
11. In the synthesis of sodium carbonate, the recovery of ammonia is done by treating $\mathrm{NH}_{4} \mathrm{Cl}$ with $\mathrm{Ca}(\mathrm{OH})_{2}$ " The by-product obtained in this process is
(A) $\mathrm{CaCl}_{2}$
(B) $\mathrm{NaCl}$
(C) $\mathrm{NaOH}$
(D) $\mathrm{NaHCO}_{3}$
Ans: Correct option(A)
Explanation:
Sodium carbonate is prepared by Solvay's process. In this process, ammonia is recovered when ammonium chloride reacts with calcium hydroxide. On reacting $\mathrm{NH}_{4} \mathrm{Cl}$ with $Ca(OH)_{2}$ calcium chloride is obtained as a by-product. The reaction is given below.
$2 \mathrm{NH}_{4} \mathrm{Cl}+\mathrm{Ca}(\mathrm{OH})_{2} \rightarrow 2 \mathrm{NH}_{3}+\mathrm{CaCl}_{2}+\mathrm{H}_{2} \mathrm{O}$
12. When sodium is dissolved in liquid ammonia, a solution of deep blue colour is obtained. The color of the solution is due to
(A) ammoniated electron
(B) sodium ion
(C) sodium amide
(D) ammoniated sodium ion
Ans: Correct option(A)
Explanation:
The alkali metals dissolve in liquid ammonia and give a blue solution, which is conductive in nature. A solution of sodium in liquid ammonia at $-30^{\circ} \mathrm{C}$ conducts electricity. The ammoniated electrons are responsible for the blue color of the solution as they absorb energy in the visible region of light and impart blue color to the solution.
13. By adding gypsum to cement
(A) setting time of cement becomes less.
(B) setting time of cement increases.
(C) colour of cement becomes light
(D) shining surface is obtained.
Ans: Correct option(B)
Explanation:
On adding water to cement it gets harder over a period of time. This is known as setting of cement. Gypsum is added to the cement to slow the setting time of cement which occurs in the presence of water.
So, option (B) is correct.
14. Dead burnt plaster is
(A) $\mathrm{CaSO}_{4}$
(B) $\mathrm{CaSO}_{4} \cdot \dfrac{1}{2} \mathrm{H}_{2} \mathrm{O}$
(C) $\mathrm{CaSO}_{4} \cdot \mathrm{H}_{2} \mathrm{O}$
(D) $\mathrm{CaSO}_{4} \cdot 2 \mathrm{H}_{2} \mathrm{O}$
Ans: Correct option(A)
Explanation:
An anhydrous calcium sulfate is dead burnt paris. It is known as dead burnt because it does not set like plaster when moistened with water. When gypsum is heated the water of crystallization is lost and an anhydrous calcium sulphate is obtained.
15. Suspension of slaked lime in water is known as
(A) Lime water
(B) Quick lime
(C) Milk of lime
(D) Aqueous solution of slaked lime
Ans: Correct Option(C)
Explanation:
$\mathrm{Ca}(\mathrm{OH})_{2}$ saked lime is only sparingly soluble in water. When $\mathrm{CaO}$ is soaked in water, it produces a white suspension, and the reaction is highly exothermic. White suspension is called milk of lime and it is used for white washing.
16. Which of the following elements does not form hydride by direct heating with dihydrogen?
(A) Be
(B) $\mathrm{Mg}$
(C) $\mathrm{Sr}$
(D) $\mathrm{Ba}$
Ans: Correct option(A)
Explanation:
Beryllium being least reactive does not form hydride by direct heating with dihydrogen. Also due to its small size and high ionization enthalpy it cannot form hydrides by direct heating. So, option (A) is correct.
17. The formula of soda ash is
(A) $\mathrm{Na}_{2} \mathrm{CO}_{3} \cdot 10 \mathrm{H}_{2} \mathrm{O}$
(B) $\mathrm{Na}_{2} \mathrm{CO}_{3} \cdot 2 \mathrm{H}_{2} \mathrm{O}$
(C) $\mathrm{Na}_{2} \mathrm{CO}_{3} \cdot \mathrm{H}_{2} \mathrm{O}$
(D) $\mathrm{Na}_{2} \mathrm{CO}_{3}$
Ans: Correct option(D)
Explanation:
Anhydrous sodium carbonate is known as soda ash. The formula of soda ash is $\mathrm{Na}_{2} \mathrm{CO}_{3} \cdot$ It is also known as washing soda. So, option (D) is correct.
18. A substance which gives brick red flame and breaks down on heating to give oxygen and a brown gas is
(A) Magnesium nitrate
(B) Calcium nitrate
(C) Barium nitrate
(D) Strontium nitrate
Ans: Correct option(B)
Explanation:
Alkali and alkaline metals impart characteristic color. Calcium gives brick red color, Strontium gives crimson red color, Barium gives apple green color, and radium gives crimson color. So, the compound is calcium nitrate. On heating calcium nitrate, it gives calcium oxide, nitrogen and nitrogen dioxide gas which is brown in color.
$\mathrm{Ca}\left(\mathrm{NO}_{3}\right)_{2} \rightarrow \mathrm{CaO}+\mathrm{O}_{2}+\mathrm{NO}_{2} \uparrow$
19. Which of the following statements is true about $\mathrm{Ca}(\mathrm{OH})_{2}$ ?
(A) It is used in the preparation of bleaching powder.
(B) It is a light blue solid.
(C) It does not possess disinfectant property.
(D) It is used in the manufacture of cement.
Ans: Correct option(A)
Explanation:
Calcium hydroxide is used in the preparation of bleaching powder. It reacts with chlorine to form hypochlorite, a constituent of bleaching powder.
$\mathrm{Ca}(\mathrm{OH})_{2}+\mathrm{Cl}_{2} \rightarrow \mathrm{CaCl}_{2}+\mathrm{H}_{2} \mathrm{O}+\mathrm{Ca}(\mathrm{OCl})_{2}$
It is a white amorphous powder and is sparingly soluble in water.
It possess disinfectant property and therefore, is used in white ash.
Cement is made from calcium oxide and the addition of other material such as clay, silica.
20. A chemical $A$ is used for the preparation of washing soda to recover ammonia. When $C O_{2}$ is bubbled through an aqueous solution of $A$, the solution turns milky. It is used in white washing due to disinfectant nature. What is the chemical formula of $A$ ?
(A) $\mathrm{Ca}\left(\mathrm{HCO}_{3}\right)_{2}$
(B) $\mathrm{CaO}$
(C) $\mathrm{Ca}(\mathrm{OH})_{2}$
(D) $\mathrm{CaCO}_{3}$
Ans: Correct Option(C)
Explanation:
The chemical formula of $\mathrm{A}$ is $\mathrm{Ca}(\mathrm{OH})_{2}{ }^{\circ}$ Sodium carbonate is prepared by Solvay's process. In this process, ammonia is recovered when ammonium chloride reacts with calcium hydroxide. On reacting
$\mathrm{NH}_{4} \mathrm{Cl}$ with $ \mathrm{Ca}(\mathrm{OH})_{2}$, calcium chloride is obtained as a by-product. The reaction is given below.
$2 \mathrm{NH}_{4} \mathrm{Cl}+\mathrm{Ca}(\mathrm{OH})_{2} \rightarrow 2 \mathrm{NH}_{3}+\mathrm{CaCl}_{2}+\mathrm{H}_{2} \mathrm{O}$
When $\mathrm{CO}_{2}$ is bubbled through an aqueous solution of $\mathrm{Ca}(\mathrm{OH})_{2}$ the solution turns milky. It is used in whitewashing due to its disinfectant nature.
21. Dehydration of hydrates of halides of calcium, barium and strontium i.e., $\mathrm{CaCl}_{2} \cdot 6 \mathrm{H}_{2} \mathrm{O}$, $\mathrm{BaCl}_{2} \cdot 2 \mathrm{H}_{2} \mathrm{O}, \mathrm{SrCl}_{2} \cdot 2 \mathrm{H}_{2} \mathrm{O}$ can be achieved by heating. These become wet on keeping in the air. Which of the following statements is correct about these halides?
(A) act as dehydrating agent
(B) can absorb moisture from air
(C) Tendency to form hydrate decreases from calcium to barium
(D) All of the above
Ans: Correct option(D)
Explanation:
Chlorides of alkaline earth metals are hydrated salts. They can be used as dehydrating agents due to their hygroscopic nature to absorb moisture from air. Extent of hydration decreases from Mg to Ba.
Therefore, dehydration of hydrates of halides of calcium, barium and strontium i.e., $\mathrm{CaCl}_{2} .6 \mathrm{H}_{2} \mathrm{O}$, $\mathrm{BaCl}_{2} \cdot 2 \mathrm{H}_{2} \mathrm{O}, \mathrm{SrCl}_{2} \cdot 2 \mathrm{H}_{2} \mathrm{O}$ can be achieved by heating.
II. Multiple Choice Questions (Type-II)
22. Metallic elements are described by their standard electrode potential, fusion enthalpy, atomic size, etc. The alkali metals are characterised by which of the following properties?
(A) High boiling point
(B) High negative standard electrode potential
(C) High density
(D) Large atomic size
Ans: Correct option(B) and (D)
Explanation:
Alkali metals have larger atomic size and low density. These metals lose electrons due to less effective nuclear charge. They have a high value of electrode potential.
Lithium has the highest negative reduction potential value. Due to the small size of lithium it has a small atomic size and highest ionization enthalpy.
23. Several sodium compounds find use in industries. Which of the following compounds are used for the textile industry?
(A) $\mathrm{Na}_{2} \mathrm{CO}_{3}$
(B) $\mathrm{NaHCO}_{3}$
(C) $\mathrm{NaOH}$
(D) $\mathrm{NaCl}$
Ans: Correct option(A) and (C)
Explanation:
$\mathrm{Na}_{2} \mathrm{CO}_{3}$ is used in paper paints and textile industry. It is used to manufacture soap powders.
$\mathrm{NaOH}$ is used in textile industry for mercerising cotton fabrics.
24. Which of the following compounds are readily soluble in water?
(A) $\mathrm{BeSO}_{4}$
(B) $\mathrm{MgSO}_{4}$
(C) $\mathrm{BaSO}_{4}$
(D) $\mathrm{SrSO}_{4}$
Ans: Correct option (A) and (B)
Explanation:
On moving down the group, as the size increases the hydration enthalpy decreases. The hydration enthalpy of beryllium and magnesium ions are high due to the small size. Thus, sulfates are readily soluble in water.
25. When Zeolite, which is hydrated sodium aluminium silicate, is treated with hard water, the sodium ions are exchanged with which of the following ion(s)?
(A) $\mathrm{H}^{+}$ions
(B) $\mathrm{Mg}^{2+}$ ions
(C) $\mathrm{Ca}^{2+}$ ions
(D) $\mathrm{SO}_{4}^{2-}$ ions
Ans: Correct option (B) and (C)
Explanation:
Zeolite is sodium aluminum silicate. It is used to reduce the hardness of water. It has a property to exchange $\mathrm{Mg}^{2+}$ ions and $\mathrm{Ca}^{2+}$ ions from hard water by the $\mathrm{Na}^{+}$ions of zeolite.
26. Identify the correct formula of halides of alkaline earth metals from the following.
(A) $\mathrm{BaCl}_{2} \cdot 2 \mathrm{H}_{2} \mathrm{O}$
(B) $\mathrm{BaCl}_{2} \cdot 4 \mathrm{H}_{2} \mathrm{O}$
(C) $\mathrm{CaCl}_{2} \cdot 6 \mathrm{H}_{2} \mathrm{O}$
(D) $\mathrm{SrCl}_{2} \cdot 4 \mathrm{H}_{2} \mathrm{O}$
Ans: Correct option(A) and (C)
Explanation:
The chlorides of alklaje earth metals are hydrated. On moving down the group, the extent of hydration decreases. Therefore, the correct formula of halides are: $\mathrm{CaCl}_{2} \cdot 6 \mathrm{H}_{2} \mathrm{O}$ and $\mathrm{BaCl}_{2} \cdot 2 \mathrm{H}_{2} \mathrm{O}$.
27. Choose the correct statements from the following.
(A) Beryllium is not readily attacked by acids because of the presence of an oxide film on the surface of the metal.
(B) Beryllium sulphate is readily soluble in water as the greater hydration enthalpy of $\mathrm{Be}^{2+}$ overcomes the lattice enthalpy factor.
(C) Beryllium exhibits coordination number more than four.
(D) Beryllium oxide is purely acidic in nature.
Ans: Correct option(A) and (B)
Explanation:
Beryllium resembles aluminum through the diagonal relation. Beryllium forms a protective film of oxide on the surface, and thus, is prevented by the attack of acids.
Thus, statement (1) is correct.
On moving down the group, as the size increases the hydration enthalpy decreases. The hydration enthalpy of beryllium ions are high due to the small size. Thus, sulfates are readily soluble in water. Thus, statement (2) is correct.
Beryllium does not exhibit coordination numbers more than four. As it has no $d$ orbitals.
Thus, statement (3) is not correct.
Beryllium oxide is amphoteric in nature. It reacts with both acids and bases. It reacts with acid to form beryllium chloride and it reacts with base to form becyllate ion which is soluble in sodium hydroxide. The reaction is shown below.
$\mathrm{Be}(\mathrm{OH})_{2}+2 \mathrm{OH}^{-} \rightarrow\left[\mathrm{Be}(\mathrm{OH})_{4}\right]^{2-}$
$\mathrm{Be}(\mathrm{OH})_{2}+2 \mathrm{HCl} \rightarrow \mathrm{BeCl}_{2}+2 \mathrm{H}_{2} \mathrm{O}$
Thus, statement (4) is not correct.
28. Which of the following are the correct reasons for anomalous behaviour of lithium?
(A) Exceptionally small size of its atom
(B) Its high polarising power
(C) It has high degree of hydration
(D) Exceptionally low jonisation enthalpy
Ans: Correct option(A) and (B)
Explanation:
Among the alkali metals, lithium has an exceptionally small size. Due to the small size of lithium and high nuclear charge it has high palajising power. Hence, options (A) and (B) are correct.
III. Short Answer Type
29. How do you account for the strong reducing power of lithium in aqueous solution?
Ans: Lithium has the highest negative reduction potential value. Due to the small size of lithium it has the highest ionization enthalpy. Due to this, the reducing power of lithium is highest in an aqueous solution.
30. When heated in air, the alkali metals form various oxides. Mention the oxides formed by Li, Na and $\mathrm{K}$.
Ans: Alkali metals forms oxide when reacted with air. Lithium forms monoxide, sodium forms peroxide and potassium forms superoxide.
$\mathrm{4Li}+\mathrm{O}_{2} \rightarrow \mathrm2{Li}_{2} \mathrm{O}$
$\mathrm{Na}+\mathrm{O}_{2} \rightarrow \mathrm{Na}_{2} \mathrm{O}_{2}$
$\mathrm{~K}+\mathrm{O}_{2} \rightarrow \mathrm{KO}_{2}$
31. Complete the following reactions
(j) $\mathrm{O}_{2}^{2-}+\mathrm{H}_{2} \mathrm{O} \rightarrow$
Ans: $\mathrm{O}_{2}^{2-}+2 \mathrm{H}_{2} \mathrm{O} \rightarrow \mathrm{H}_{2} \mathrm{O}_{2}+2 \mathrm{OH}^{-}$
(ii) $\mathrm{O}_{2}^{-}+\mathrm{H}_{2} \mathrm{O} \rightarrow$
Ans: $2 \mathrm{O}_{2}^{-}+2 \mathrm{H}_{2} \mathrm{O} \rightarrow 2 \mathrm{OH}^{-}+\mathrm{H}_{2} \mathrm{O}_{2}+\mathrm{O}_{2}$
32. Lithium resembles magnesium in some of its properties. Mention two such properties and give reasons for this resemblance.
Ans: These two elements have similar properties because of their similar atomic and ionic radii.
(i) Both are lighter element and harder than the other metals in their respective groups.
(ii) The halides of both elements, $\mathrm{LiCl}$ and $\mathrm{MgCl}_{2}$ are soluble in ethanol.
33. Name an element from Group 2 which forms an amphoteric oxide and a water soluble sulphate,
Ans: The element from group 2 is beryllium. $\operatorname{Be}(\mathrm{OH})_{2}$ is amphoteric. It reacts with both acids and bases. It reacts with acid to form beryllium chloride and it reacts with base to form beryllate ion which is soluble in sodium hydroxide. The reaction is shown below.
$\mathrm{Be}(\mathrm{OH})_{2}+2 \mathrm{OH}^{-} \rightarrow\left[\mathrm{Be}(\mathrm{OH})_{4}\right]^{2-}$
$\mathrm{Be}(\mathrm{OH})_{2}+2 \mathrm{HCl} \rightarrow \mathrm{BeCl}_{2}+2 \mathrm{H}_{2} \mathrm{O}$
Beryllium sulfate are readily soluble in water.
34. Discuss the trend of the following:
(i) Thermal stability of carbonates of Group 2 elements.
(ii) The solubility and the nature of oxides of Group 2 elements.
Ans: (i) As the size of the cation increases, the thermal stability of carbonate increases. The more stable will be the oxide of an alkaline earth metal, the less stable will be the carbonate. Hence, beryllium carbonate will be highly unstable because its oxide will be stable.
(ii) Alkali metals and alkaline earth metals form oxides with oxygen and give metal oxides. The oxides are basic in nature. $\mathrm{BeO}$ is an exception because $\mathrm{BeO}$ is amphoteric.
They also react with water to form sparingly soluble hydroxides. On increasing the size of the cations beryllium oxide and magnesium oxide have the highest lattice energy and they are insoluble in water.
35. Why are $\mathrm{BeSO}_{4}$ and $\mathrm{MgSO}_{4}$ readily soluble in water while $\mathrm{CaSO}_{4}, \mathrm{SrSO}_{4}$ and $\mathrm{BaSO}_{4}$ are insoluble?
Ans: $\mathrm{BeSO}_{4}$ and $\mathrm{MgSO}_{4}$ readily soluble in water while $\mathrm{CaSO}_{4}, \mathrm{SrSO}_{4}$ and $\mathrm{BaSO}_{4}$ are insoluble The greater hydration enthalpy of $\mathrm{Be}^{2+}$ and $\mathrm{Mg}^{2+}$ ions overcome the lattice enthalpy factor and therefore, their sulfates are soluble.
36. All compounds of alkali metals are easily soluble in water but lithium compounds are more soluble in organic solvents. Explain.
Ans: Ionic compounds are formed from the alkali metals due to their large ionic size and low ionization enthalpy. Thus, they are soluble in water. But, due to the small ionic size, high ionization enthalpy, and high electronegativity of lithium, it forms compounds of covalent nature and thus, are soluble in organic solvents.
37. In the Solvay process, can we obtain sodium carbonate directly by treating the solution containing $\left(\mathrm{NH}_{4}\right)_{2} \mathrm{CO}_{3}$ with sodium chloride? Explain.
Ans: In the Solvay process, carbon dioxide is transferred through a concentrated solution of ammoniacontaining sodium chloride, which forms ammonium carbonate followed by ammonium hydrogen carbonate. The chemicals in ammonium hydrogen carbonate are different and are heated to form sodium carbonate. $\mathrm{NH}_{3}$ is found in a solution containing $\mathrm{NH}_{4} \mathrm{Cl}$ that is heated and treated with $\mathrm{Ca}(\mathrm{OH})_{2}{ }^{*}$ The reaction of $\left(\mathrm{NH}_{4}\right)_{2} \mathrm{CO}_{3}$ with $\mathrm{NaCl}$ provides two products, $\mathrm{Na}_{2} \mathrm{CO}_{3}$ and $\mathrm{NH}_{4} \mathrm{Cl}$ both soluble in water which do not shift to the right balance.
38. Write the Lewis structure of $\mathrm{O}^{2-}$ ion and find out the oxidation state of each oxygen atom? What is the average oxidation state of oxygen in this ion?
Ans: The lewis structure of $\mathrm{O}^{2-}$ ion is,
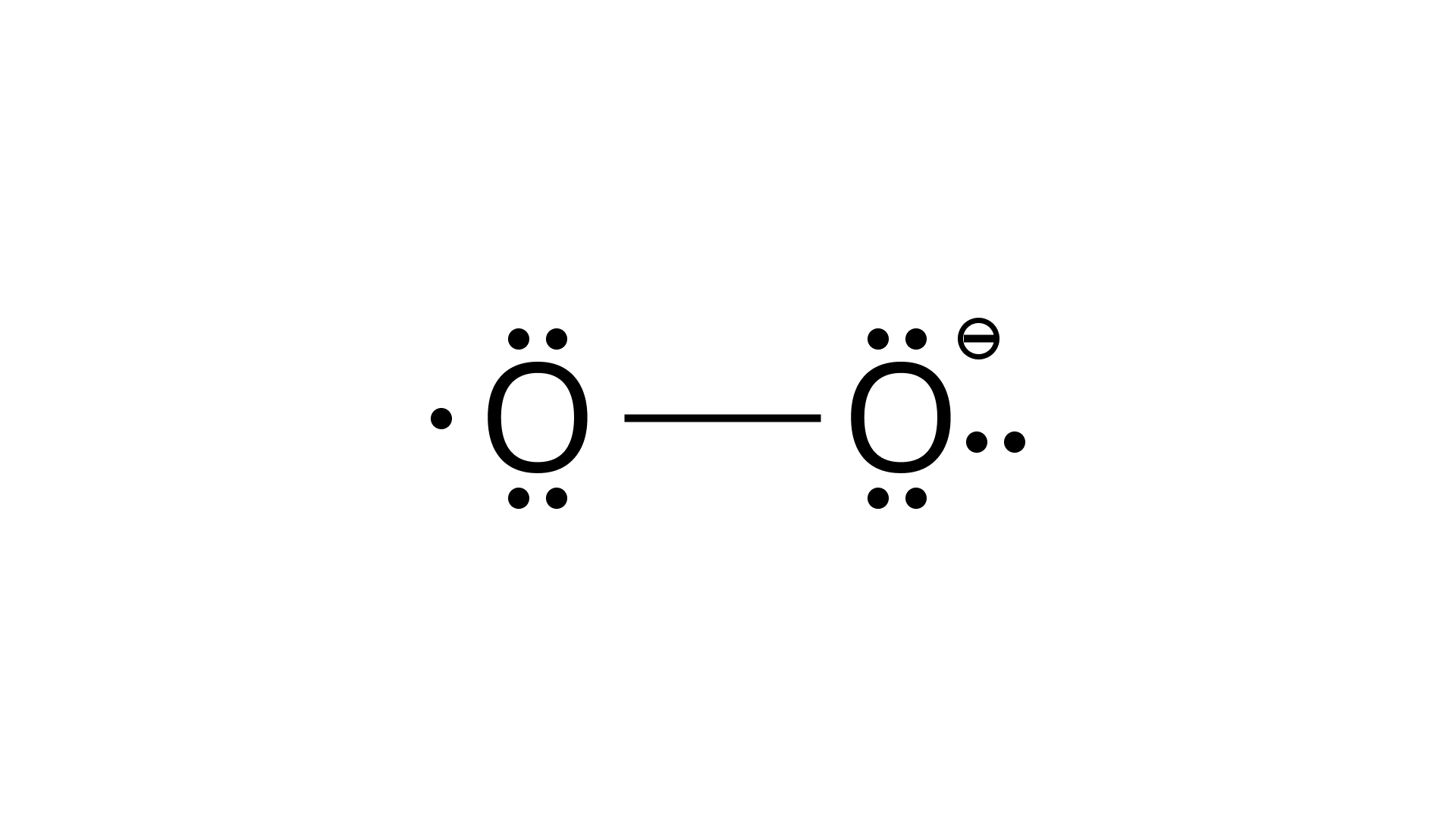
Oxygen atom having no charge has 6 electrons, so its oxidation number is zero. Oxygen atoms containing $-1$ charge have 7 electrons, so its oxidation number is $-1$. The average oxidation state of oxygen in this ion is,
$=\dfrac{1}{2}$
39. Why do beryllium and magnesium not impart coleucto the flame in the flame test?
Ans: The flame is due to the excitation of the electron from its higher energy state to the lower energy states. Due to the small atomic and ionic size of beryllium and magnesium, electrons are tightly bound to the atom. The electrons of Be and Mg does not gain excitation from the energy provided by the flame. Hence they do not show any flame in the flame test.
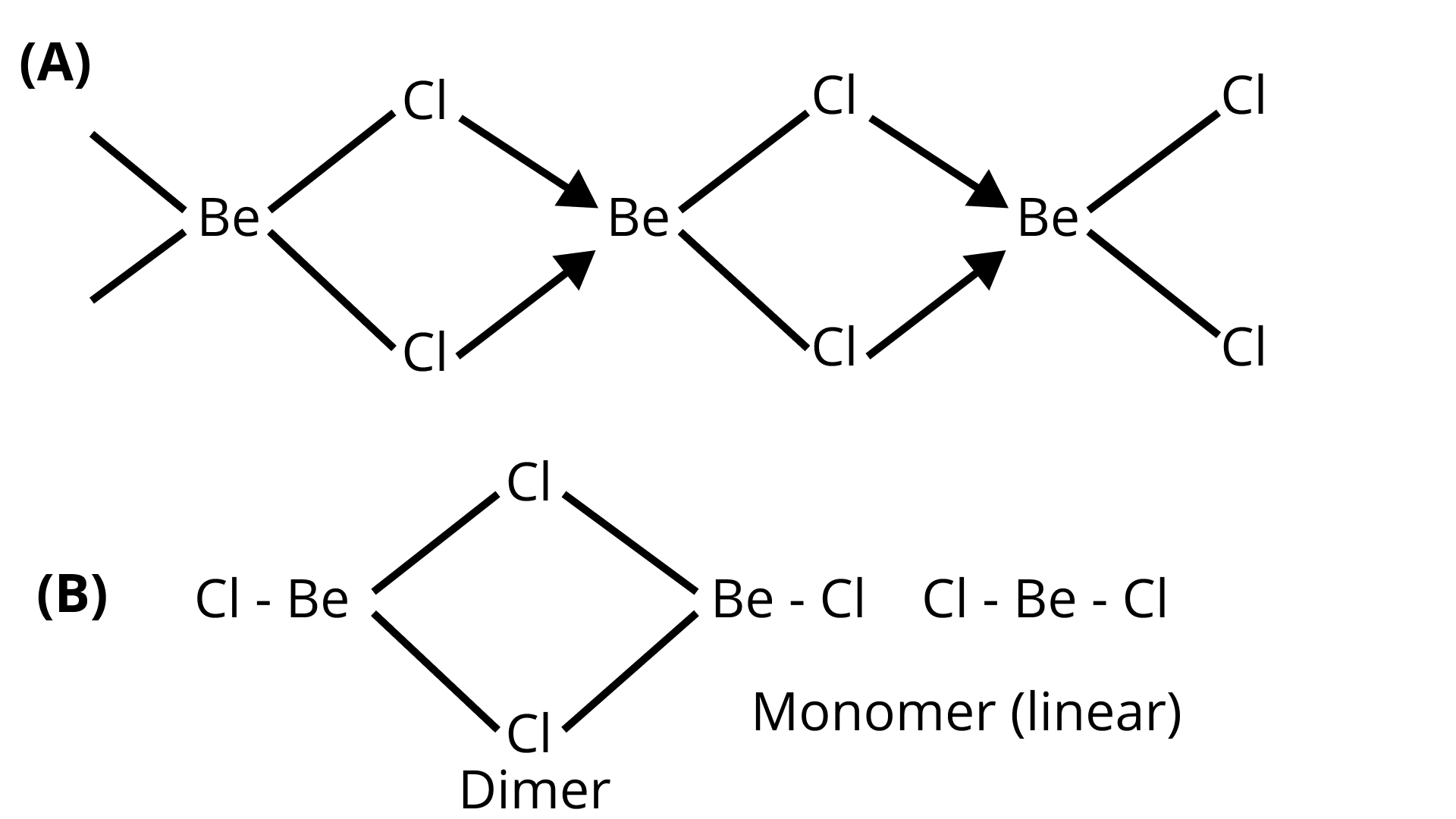
40. What is the structure of $\mathrm{BeCl}_{2}$ molecule in gaseous and solid state?
Ans: In the solid state $\mathrm{BeCl}_{2}$ has a polymeric chain like structure. Be atom is surrounded by four $\mathrm{Cl}$ atoms among which two of them are bonded through covalent bond and other two are through co-ordinate bond. Structure of $\mathrm{BeCl}_{2}$ is given by structure $\mathrm{A}$.
In gaseous state beryllium chloride exists as $\operatorname{dimer}\left(\mathrm{Be}_{2} \mathrm{Cl}_{4}\right)$ which dissociates to the monomer at about $1200 \mathrm{~K}$ temperature which is linear in structure.
Structure of $\mathrm{BeCl}_{2}$ in gaseous state is given by the structure $\mathrm{B}$.
IV. Matching Type
41. Match the elements given in Column I with the properties mentioned in Column II.
Column I | Column II |
(i) Li | (a) Insoluble sulphate |
(ii) Na | (b) Strongest monoclinic base |
(iii) Ca | (c) Most negative Eo value among alkali metals |
(iv) Ba | (d) Insoluble oxalate |
(e) 6s2 outer electronic configuration |
Ans:
(i) Lithium has the highest negative reduction potential value. Due to the small size of lithium it has a small atomic size, the highest ionization enthalpy Due to this, the reducing power of lithium is highest.
Thus, option (i) from column I is matched with (c) from column II.
(ii) Sodium is a stronger monoacidic base due to lower first ionization enthalpy of sodium. Also sodium forms sodium hydroxide Therefore 1 mole of it replaces one mole of hydrogen ions from acids.
Thus, option (ii) from column I is matched with (b) from column II.
(iii) Calcium oxalate is an ionic compound because its hydration enthalpy is low. It is highly insoluble and dissolves poorly in water.
Thus, option (iii) from column I is matched with (d) from column II.
(iv) Barium sulfate is insoluble due to the high hydration energy. The electronic configuration of barium is $1 s^{2} 2 s^{2} 2 p^{6} 3 s^{2} 3 p^{6} 3 d^{10} 4 s^{2} 4 p^{6} 4 d^{10} 5 s^{2} 5 p^{6} 6 s^{2}$
Thus, option (iv) from column I is matched with (a) and (e) from column II.
42. Match the compounds given in Column I with their uses mentioned in Column II.
Column I | Column II |
(i) CaCO3 | (a) Density, ornamental work |
(ii) Ca(OH)2 | (b) Manufacture of sodium carbonate from caustic soda |
(iii) CaO | (c) Manufacture of high quality paper |
(iv) CaSO4 | (d) Used in white washing |
Ans: (i) $\mathrm{CaCO}_{3}$ is used in manufacturing of high quality paper.
Thus, option (i) from column I is matched with (c) from column II.
(ii)Due to the disinfectant nature of $\mathrm{Ca}(\mathrm{OH})_{2}$ is used in white wash.
Thus, option (ii) from column $\mathrm{l}$ is matched with (d) from column II.
(iii) $\mathrm{CaO}$ is used in the manufacture of sodium carbonate from caustic soda.
Thus, option (iii) from column I is matched with (b) from column II.
(iv) $\mathrm{CaSO}_{4}$ is used in dentistry, ornamental work and for making statues.
Thus, option (iv) from column I is matched with (a) from column II.
43. Match the elements given in Column I with the colour they impart to the flame given in Column II.
Column I | Column II |
(i) Cs | (a) Apple green |
(ii) Na | (b) Violet |
(iii) K | (c) Brick red |
(iv) Ca | (d) Yellow |
(v) Sr | (e) Crimson red |
(vi) Ba | (f) Blue |
Ans: All alkali metal and alkaline earth metals except beryllium and magnesium gives characteristic color when introduced into flame. Due to released energy being absorbed in the visible region, alkali metals and alkaline earth metals gives characteristic color.
(i) Caesium imparts blue color when introduced into flame.
Thus, option (i) from column I is matched with (f) from column II.
(ii) Sodium imparts yellow color when introduced into flame.
Thus, option (i) from column I is matched with (d) from column II.
(iii) Potassium imparts violet color when introduced into flame.
Thus, option (i) from column I is matched with (b) from column II.
(iv) Calcium imparts brick red color when introduced into flame.
Thus, option (i) from column I is matched with (c) from column II.
(v) Strontium imparts crimson red color when introduced into flame.
Thus, option (i) from column I is matched with (e) from column II.
(vi) Barium imparts apple green color when introduced into flame.
Thus, option (i) from column I is matched with (a) from column II.
V. Assertion and Reason Type
44. Assertion (A): The carbonate of lithium decomposes easily on heating to form lithium oxide and $\mathrm{CO}_{2} \cdot$ Reason (R): Lithium being very small in size polarises large carbonate ion leading to the formation of more stable $\mathrm{Li}_{2} \mathrm{O}$ and $\mathrm{CO}_{2}$.
(A) Both $A$ and $R$ are correct and $R$ is the correct explanation of $A$.
(B) Both $A$ and $R$ are correct but $R$ is not the correct explanation of $A$.
(C) Both $A$ and $R$ are not correct.
(D) $A$ is not correct but $R$ is correct.
Ans: (A)
Explanation:
Lithium beimg small in size, polarises large carbonate ion. Polarisation is the distortion of electron cloud of the anion by the cation. Thus, the carbonate of lithium decomposes easily on heating to form lithium oxide and $\mathrm{CO}_{2}$. The reaction is shown below.
$\mathrm{Li}_{2} \mathrm{CO}_{3} \rightleftharpoons \mathrm{Li}_{2} \mathrm{O}+\mathrm{CO}_{2}$
45. Assertion (A): Beryllium carbonate is kept in the atmosphere of carbon dioxide. Reason $(R):$ Beryllium carbonate is unstable and decomposes to give beryllium oxide and carbon dioxide.
(A) Both $A$ and $R$ are correct and $R$ is the correct explanation of $A$.
(B) Both $A$ and $R$ are correct but $R$ is not the correct explanation of $A$.
(C) Both $\mathrm{A}$ and $\mathrm{R}$ are not correct.
(D) $A$ is not correct but $R$ is correct.
Ans: (A)
Explanation:
Alkaline earth metals are stable at room temperature except beryllium carbonate. It decomposes to give beryllium oxide and carbon dioxide. It is kept in an atmosphere of carbon dioxide so that equilibrium shifts to the right. The reaction is shown below. $\mathrm{BeCO}_{3} \rightleftharpoons \mathrm{BeO}+\mathrm{CO}_{2}$
Vi. Long Answer Type
46. The s-block elements are characterised by their larger atomic sizes, lower ionisation enthalpies, invariable $+1$ oxidation state and solubilities of their oxosalts. In the light of these features describe the nature of their oxides, halides and oxosalts. Ans: Alkali metals are ionic in nature due to their larger size. They have $+1$ oxidation states. Alkali metals forms three types of oxides such as peroxides, superoxides and normal oxides. The basic character of normal oxides increases from lithium oxide to caesium oxide.
The halides of alkali metals are also ionic except lithium halide. Lithium halide is covalent in nature because of small size and high polarizing power.
Oxosalts of alkali metal are solid water-soluble ionic compounds. Oxosalts of lithium show different properties due to small size of lithium.
47. Present a comparative account of the alkali and alkaline earth metals with respect to the following characteristics: (i) Tendency to form ionic / covalent compounds (ii) Nature of oxides and their solubility in water (iii) Formation of oxosalts (iv) Solubility of oxosalts (v) Thermal stability of oxosalts
Ans: (i) Alkaline earth metal compounds are less ionic than alkali metals because of small size and more effective nuclear charge.
(ii) Oxides of alkali metals are more basic than alkaline earth metals. This are water soluble and highly exothermic. The hydroxides of alkaline earth metals are less basic than alkali metals.
(iii) Alkaline earth metals give oxosalts. The reactivity of alkali metals is faster than the reactivity of alkaline earth metal. The reactivity of alkaline earth metal is less due to small size and more effective nuclear charge.
(iv) The oxo salts of alkali metals are less soluble than the oxo salts of alkaline earth metal because of small size of cation and high hydration enthalpy.
(v) The thermal stability of the oxo salts of alkali metals are more than the alkaline earth metals. The sodium carbonate is stable towards heat.
48. When a metal of group 1 was dissolved in liquid ammonia, the following observations were obtained: (i) Blue solution was obtained initially. (ii) On concentrating the solution, the blue colour changed to bronze colour. How do you account for the blue colour of the solution? Give the name of the product formed on keeping the solution for some time.
Ans: (i) The alkali metals dissolve in liquid ammonia and give a blue solution, which is conductive in nature. A solution of sodium in liquid ammonia at -30C conducts electricity. The ammoniated electrons are responsible for the blue color of the solution as they absorb energy in the visible region of light and impart blue color to the solution. Both the ammoniated cations and ammoniated electrons are responsible for the electrical conductivity of the solution.
$\mathrm{Na}+(\mathrm{x}+\mathrm{y}) \mathrm{NH}_{3} \rightarrow\left[\mathrm{Na}\left(\mathrm{NH}_{3}\right)_{\mathrm{x}}\right]^{+}+\left[\mathrm{e}\left(\mathrm{NH}_{3}\right)_{\mathrm{y}}\right]^{-}$
(ii) The blue color changes to bronze color in concentrated solution due to the formation of a cluster of metal ions. The standing blue solution liberates hydrogen gas with the formation of amide.
$\mathrm{M}^{+}+\mathrm{e}^{-}+\mathrm{NH}_{3} \rightarrow \mathrm{MNH}_{2}+\frac{1}{2} \mathrm{H}_{2}$
49. The stability of peroxide and superoxide of alkali metals increase as we go down the group. Explain giving reasons.
Ans: As the size of metal ions increases, the stability of peroxides and superoxides increases. Peroxide and superoxide ions combine with a large size of alkali metals. Lithium forms monoxide, sodium forms peroxide and potassium, rubidium and caesium forms superoxide.
$\mathrm{Li}+\mathrm{O}_{2} \rightarrow \mathrm{Li}_{2} \mathrm{O}$
$\mathrm{Na}+\mathrm{O}_{2} \rightarrow \mathrm{Na}_{2} \mathrm{O}_{2}$
$\mathrm{~K}+\mathrm{O}_{2} \rightarrow \mathrm{KO}_{2}$
50. When water is added to compound (A) of calcium, solution of compound (B) is formed. When carbon dioxide is passed into the solution, it turns milky due to the formation of compound (C). If excess carbon dioxide is passed into the solution, milkiness disappears due to the formation of compound (D). Identify the compounds $A, B, C$ and D. Explain why the milkiness disappears in the last step.
Ans: Compound A reacts with water to form compound B. So, the compound A is calcium oxide. When water is added to calcium oxide, calcium hydroxide is formed. It is lime water. The compound gives a milky appearance which is compound $\mathrm{C}$. The compound $\mathrm{C}$ is calcium carbonate. On passing, excess carbon dioxide milkiness disappears due to the formation of compound D. The compound is calcium hydrogen carbonate. The reactions are as follows:
$\mathrm{CaO}+\mathrm{H}_{2} \mathrm{O} \rightarrow \mathrm{Ca}(\mathrm{OH})_{2}$
$\mathrm{Ca}(\mathrm{OH})_{2}+\mathrm{CO}_{2} \rightarrow \mathrm{CaCO}_{3}$
$\mathrm{Ca}(\mathrm{OH})_{2}+\mathrm{CO}_{2}+\mathrm{H}_{2} \mathrm{O} \rightarrow \mathrm{Ca}\left(\mathrm{HCO}_{3}\right)_{2}$
51. Lithium hydride can be used to prepare other useful hydrides. Beryllium hydride is one of them. Suggest a route for the preparation of beryllium hydride starting from lithium hydride. Write chemical equations involved in the process.
Ans: Beryllium being least reactive does not form hydride by direct heating with dihydrogen. It is prepared by reacting it with lithium aluminum hydride. The reactions are shown below.
$8 \mathrm{LiH}+\mathrm{Al}_{2} \mathrm{Cl}_{6} \rightarrow 2 \mathrm{LiAlH}_{4}+6 \mathrm{LiCl}$
$2 \mathrm{BeCl}_{2}+\mathrm{LiAlH}_{4} \rightarrow 2 \mathrm{BeH}_{2}+\mathrm{LiCl}+\mathrm{AlCl}_{3}$
52. An element of group 2 forms covalent oxide which is amphoteric in nature and dissolves in water to give an amphoteric hydroxide. Identify the element and write chemical reactions of the hydroxide of the element with an alkali and an acid.
Ans: In group 2, only beryllium is amphoteric in nature which means it reacts with both acids and bases. Also, beryllium only forms covalent oxide due to the covalent nature. So, the element is beryllium.
It reacts with acid to form beryllium chloride and it reacts with base to form beryllate ion which is soluble in sodium hydroxide. The reaction is shown below.
$\mathrm{Be}(\mathrm{OH})_{2}+2 \mathrm{OH}^{-} \rightarrow\left[\mathrm{Be}(\mathrm{OH})_{4}\right]^{2-}$
$\mathrm{Be}(\mathrm{OH})_{2}+2 \mathrm{HCl} \rightarrow \mathrm{BeCl}_{2}+2 \mathrm{H}_{2} \mathrm{O}$
53. Ions of an element of group 1 participate in the transmission of nerve signals and transport of sugars and amino acids into cells. This element imparts yellow colouctor the flame in flame test and forms an oxide and a peroxide with oxygen. Identify the element and write a chemical reaction to show the formation of its peroxide. Why does the element impart colour to the flame?
Ans: The element imparts yellow colour to the flame in flame test which means the element of group 1 is sodium. Sodium is used in the transmission of nerve signals and transport of sugars and amino acids into cells. Reactions are shown below.
$2 \mathrm{Na}+\mathrm{O}_{2} \rightarrow \mathrm{Na}_{2} \mathrm{O}_{2}$
$4 \mathrm{Na}+\mathrm{O}_{2} \rightarrow 2 \mathrm{Na}_{2} \mathrm{O}$
$2 \mathrm{Na}_{2} \mathrm{O}+\mathrm{O}_{2} \rightarrow 2 \mathrm{Na}_{2} \mathrm{O}_{2}$
Summary of Chapter 10- S-Block Elements of Chemistry of Class 11
The periodic table is pretty long and hence for convenience sake, it has been divided into four blocks. These four blocks are s, p, d and f-blocks and are divided upon the filling of the particular shell (with electrons) similar to their names.
The very first orbital is the s-orbital. If in an element, the last electron enters the outermost orbital s-orbital, then is called the s-block element. Only the first groups (Group 1 and Group 2) of the periodic table lie in this block. Elements in these groups are called Alkali metal and Alkaline earth metals. The s-subshell can not contain more than 2 electrons in them, hence elements in these groups have 1 or 2 electrons in their outermost subshell. Group 1 has one electron and group 2 have 2 electrons in their outermost shell. These two groups combined include a total of 13 elements. Chapter 11 talks in detail about the various aspects of these 13 elements, including their chemical and physical properties.
The s-block elements are very volatile and are considered to be the most active of all metals. And thus generally not found in a free state in the natural environment. They are generally found in a combined form of halides, oxides, sulfates, carbonates, ions, etc.
Many of these 13 elements are crucial for our daily lives. The human body needs some of these elements like Sodium, potassium and calcium to work properly, their deficiency can lead to disease. Many industries are also dependent on these elements such as Lithium.
FAQs on NCERT Exemplar for Class 11 Chemistry Chapter-10 (Book Solutions)
1. How many elements are included in s-block elements? Name them.
The s-block contains a total of 13 elements in it, 7 elements from group 1 and 6 from group 2. The first seven elements of s-block are Hydrogen (H), Lithium (Li), Sodium (Na), Potassium (K), Rubidium (Rb), Cesium (Cs) and Francium (Fr), leaving hydrogen, they are called Alkali elements. The other 6 elements from group 2 are Beryllium (Be), Magnesium (Mg), Calcium (Ca), Strontium (Sr), Barium (Ba) and Radium (Ra), these are collectively called Alkaline earth metals.
2. What are the important topics and sub-topics from the Chapter 10 s-block elements?
The chapter in Chemistry entails a lot of topics and sub-topics to learn and remember, but some crucial topics need extra attention from the students. The crucial points in Chapter 10 s-block elements are listed down below -
Group 1: Alkali metals
Characteristics of Alkali metals and their compounds.
Anomalous Behavior of Lithium
Important Compounds of Sodium like salt
Importance of Sodium and Potassium in Biological process
Group 2: Alkaline metals
General properties of compounds created with alkaline earth metals.
Anomalous properties of Beryllium
Important Compound of calcium
Importance of Magnesium and Calcium in biological process
3. What are the important uses of Alkaline metals explained in Chapter 10?
Some of the important uses of alkaline metals are mentioned in Chapter 10 of Class 11 chemistry:
Uses of Lithium
Used in manufacturing of various alloys such as Lithium lead alloy.
It is used to refine copper and nickel
Uses of Sodium
It is used in the production of artificial rubber and dyes.
It is used as a reagent in many reactions to synthesis various organic compounds
Uses of Potassium
It is an important part of fertilizers used in agriculture.
Potassium has a crucial role in the biological process necessary for the existence of life.
4. Explain some Physical properties of Alkaline earth metals as mentioned in Chapter 10- s-block elements.
Physical Appearance: The alkaline earth metals are relatively soft than other metals, and have a silvery-white appearance.
Conductivities: The alkaline earth metals have a high electrical and thermal conductivity. Metals, in general, have a high electrical and thermal conductivity.
Flame Coloration: Salts of alkaline earth metals, except Be and Mg, all impart colors onto the flame. Ca gives a Brick Red color, Sr provides Crimson red, Ba gives off a grassy green and the Ra imposes a crimson red color to the flame.
5. How many questions are there in NCERT Exemplar for Class 11 Chemistry Chapter 10?
NCERT Exemplar for Class 11 Chemistry Chapter 10 s-block elements contains a total of 53 problems to be solved. These questions are related to the various concepts of the Chapter 10- s-block. The questions target various chemical and physical properties of Alkali metals and Alkaline earth metals. Students can download solutions to all these NCERT Exemplar questions from the official website of Vedantu. These questions are solved by expert chemistry teachers from Vedantu to help the student comprehend all the important concepts. The chapter-wise solutions are free to access on the website of Vedantu.

























