NCERT Exemplar for Class 11 Chemistry - Hydrocarbons - Free PDF Download
FAQs on NCERT Exemplar for Class 11 Chemistry Chapter-13 (Book Solutions)
1. What are the major classification of Hydrocarbons according to chapter 13?
Compounds containing both carbons and hydrogens are linked to each other are called hydrocarbons. Depending on the type of carbon-carbon bond present in the hydrocarbon chain, they can be classified into three major categories-
Saturated hydrocarbons: These hydrocarbons contain only a C-C single bond. There are both open and closed chain hydrocarbons. Example - Alkanes.
Unsaturated Hydrocarbons: They contain at least one C-C multiple bonds (double or triple bond). Depending upon the type of bond they can be further classified as Alkenes and Alkynes.
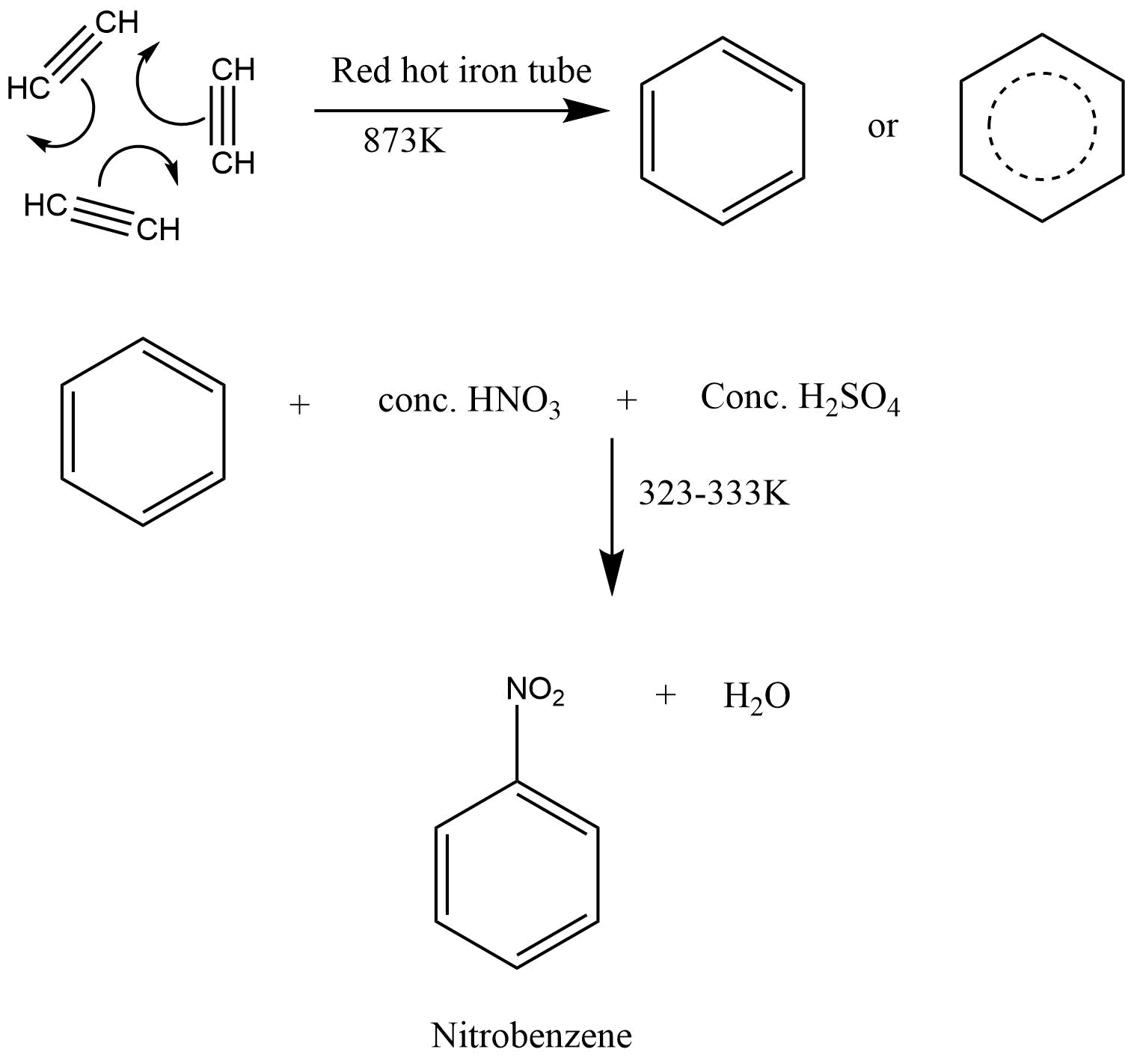
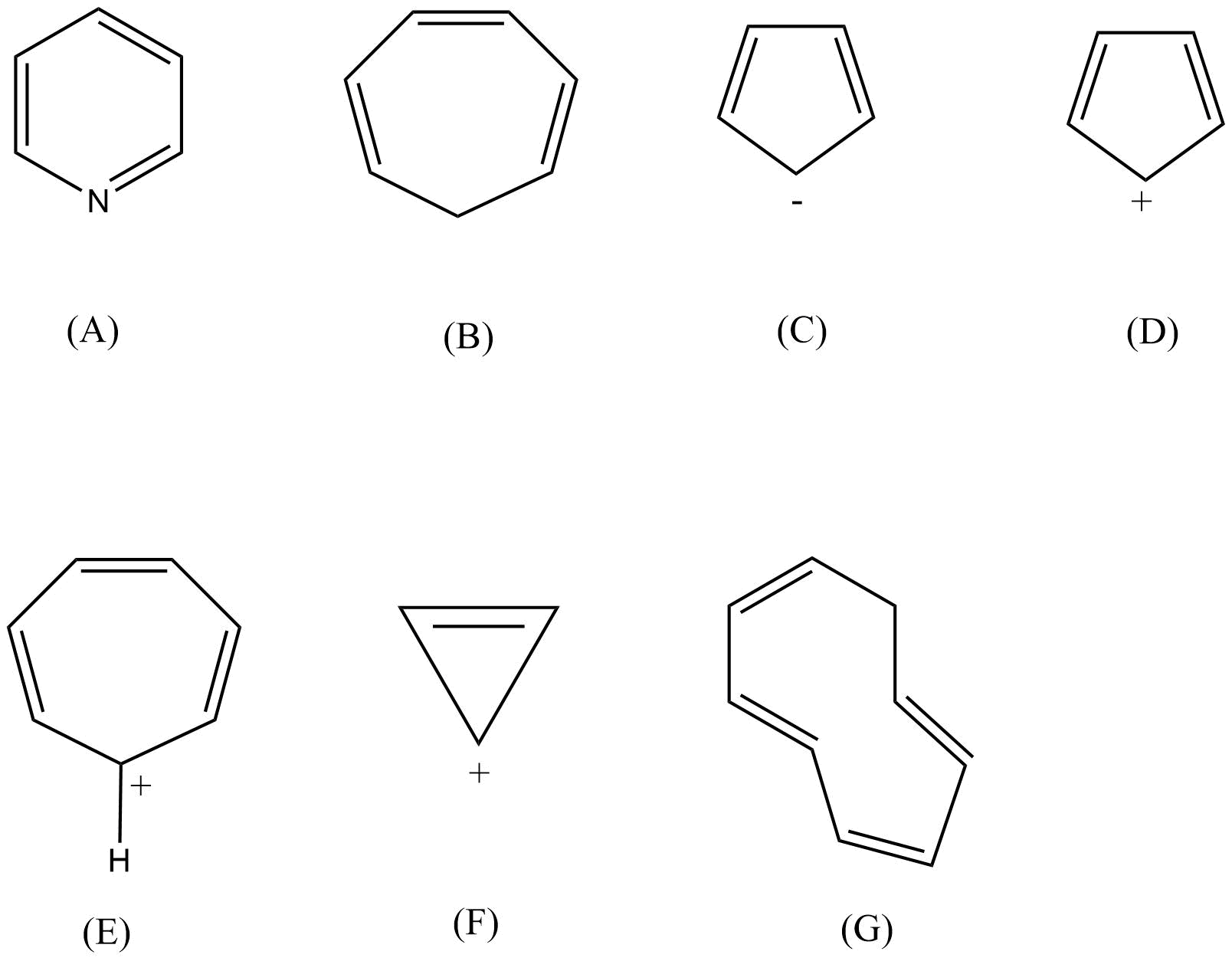
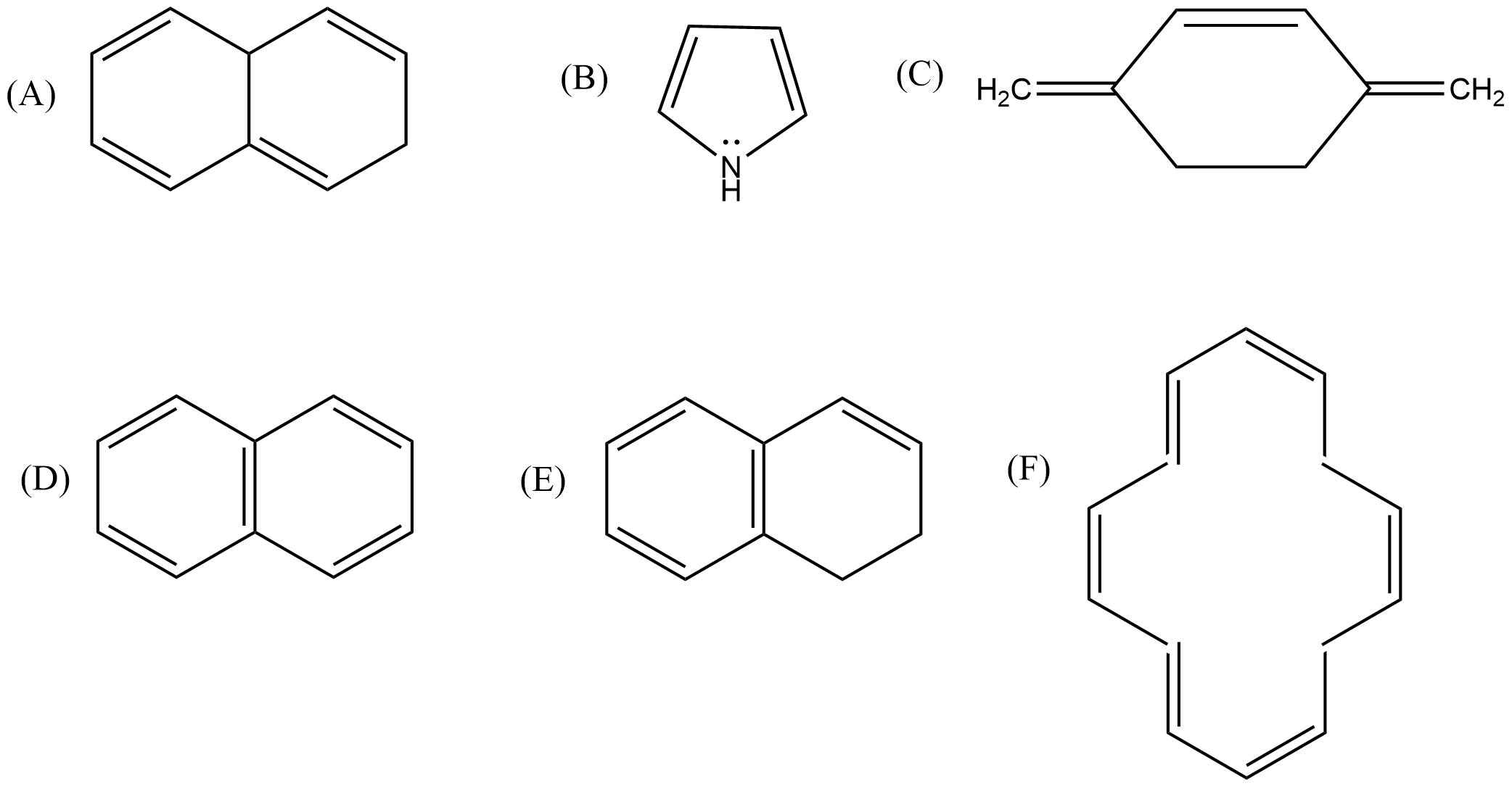
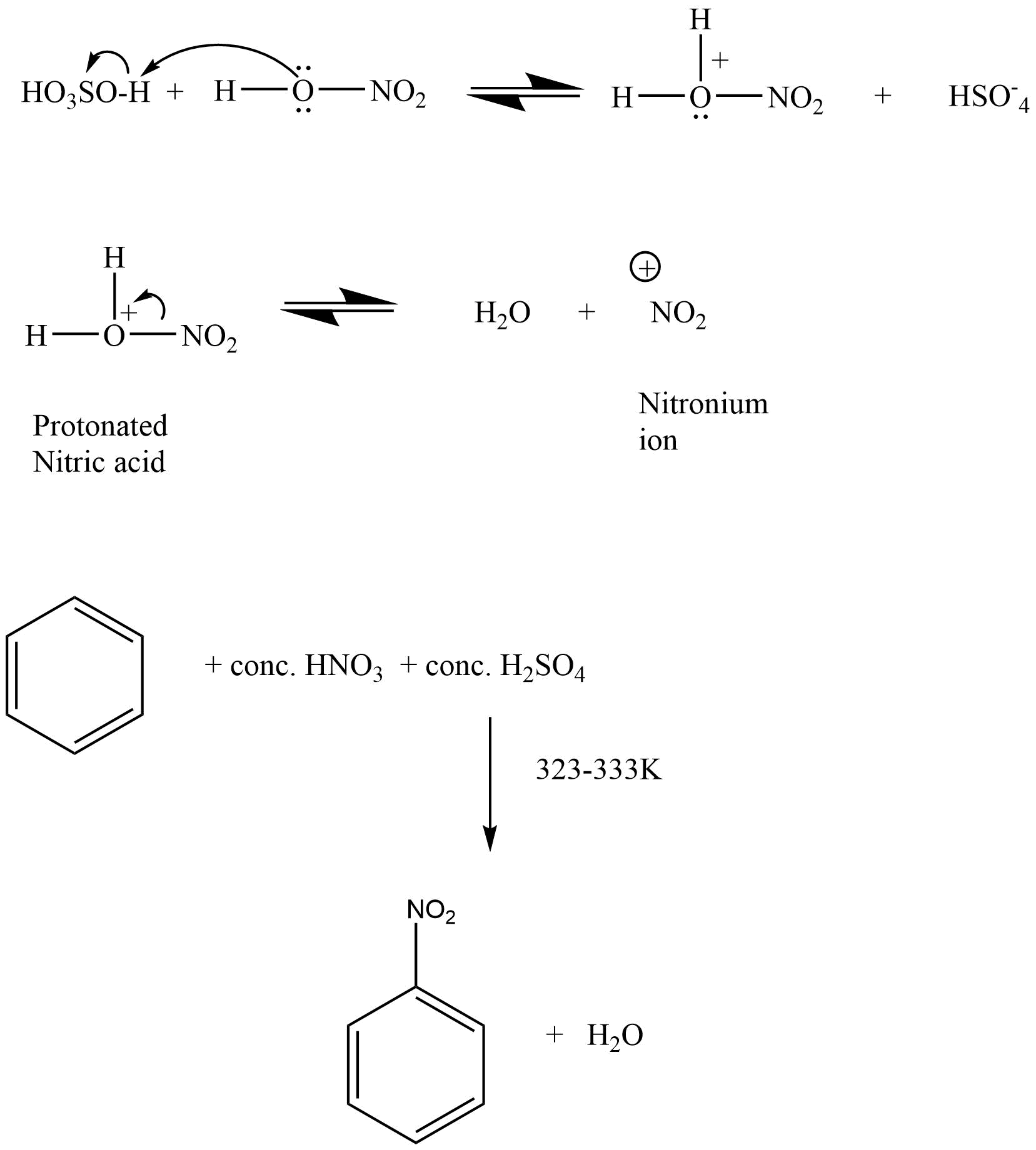
Aromatic hydrocarbons: Contains at least one special type of hexagonal ring of six carbon atoms with 3 C-C double bonds in alternate positions. An example will be benzene.
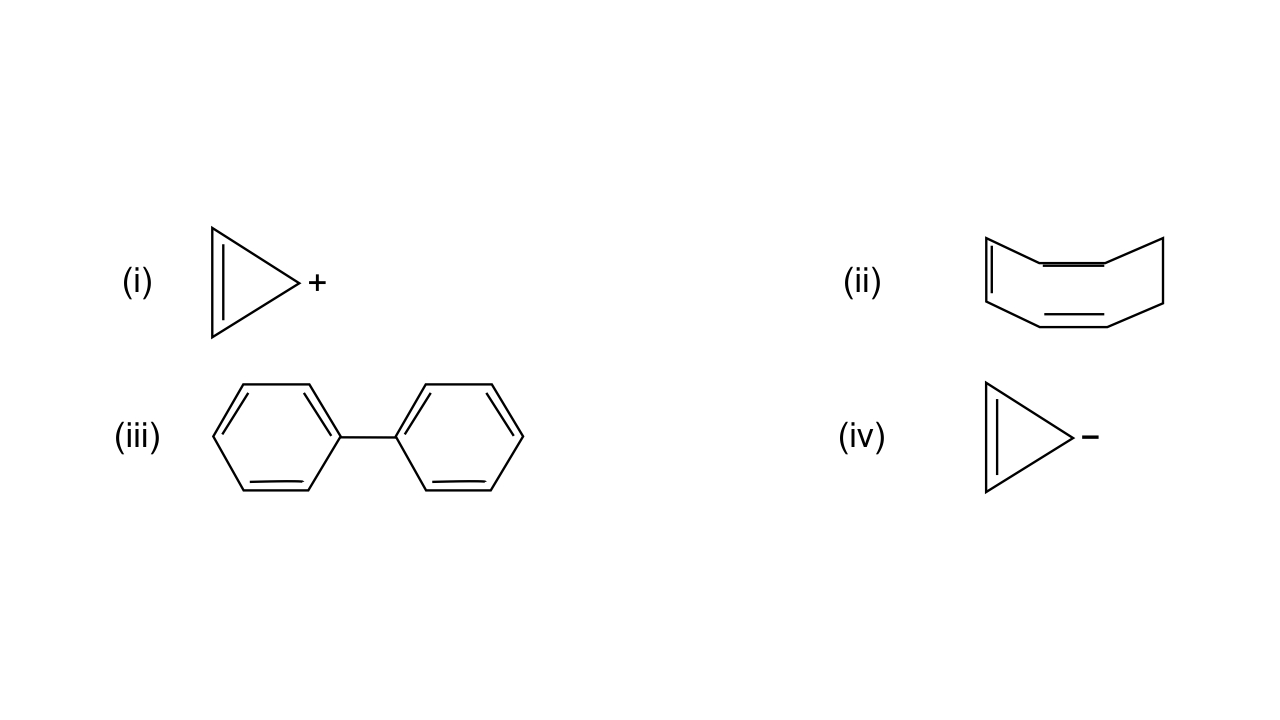
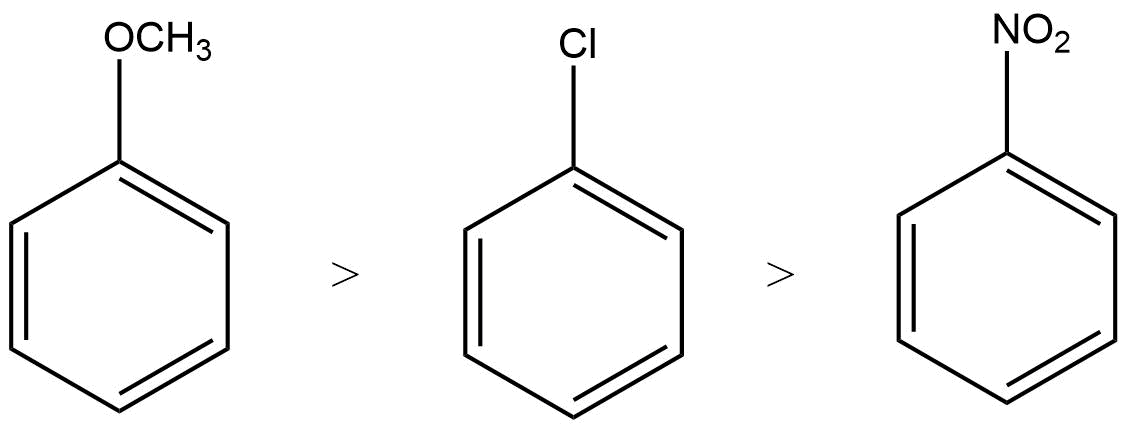
2. What are the necessary conditions for a hydrocarbon to be aromatic?
Aromatic hydrocarbons contain at least one special type of hexagonal ring of six carbon atoms with 3 C-C double bonds. Written below are some of the necessary conditions for a hydrocarbon to be aromatic-
Hydrocarbon should have a planar structure and should be cyclic.
The molecule of the hydrocarbon must contain a cloud of delocalized π-electrons above the ring.
For Aromaticity, The total number of π-electrons present in the ring should be equal to (4n+ 2), where n is an integer equal to 0, 1, 2 … etc. {Huckel’s rule}.
3. What are the properties of Benzene and its Homologues?
Following are the properties:
Benzene and its homologues, containing up to 8 carbons atoms, are colourless liquids with a distinctive smell, they appear as oils.
As aromatic hydrocarbons are like oils, they are immiscible in water but are soluble in organic solvents
They are very toxic and carcinogenic, which means they can cause cancer.
Benzene is also inflammable and burns with a yellowish sooty flame.
The melting and boiling points of aromatics substances like Benzene increases with the increase in molecular mass of the compound.
4. What are the major sub-topics from chapter 13 Hydrocarbons?
Classification of Hydrocarbons:
Alkanes
Nomenclature And Isomerism of alkanes
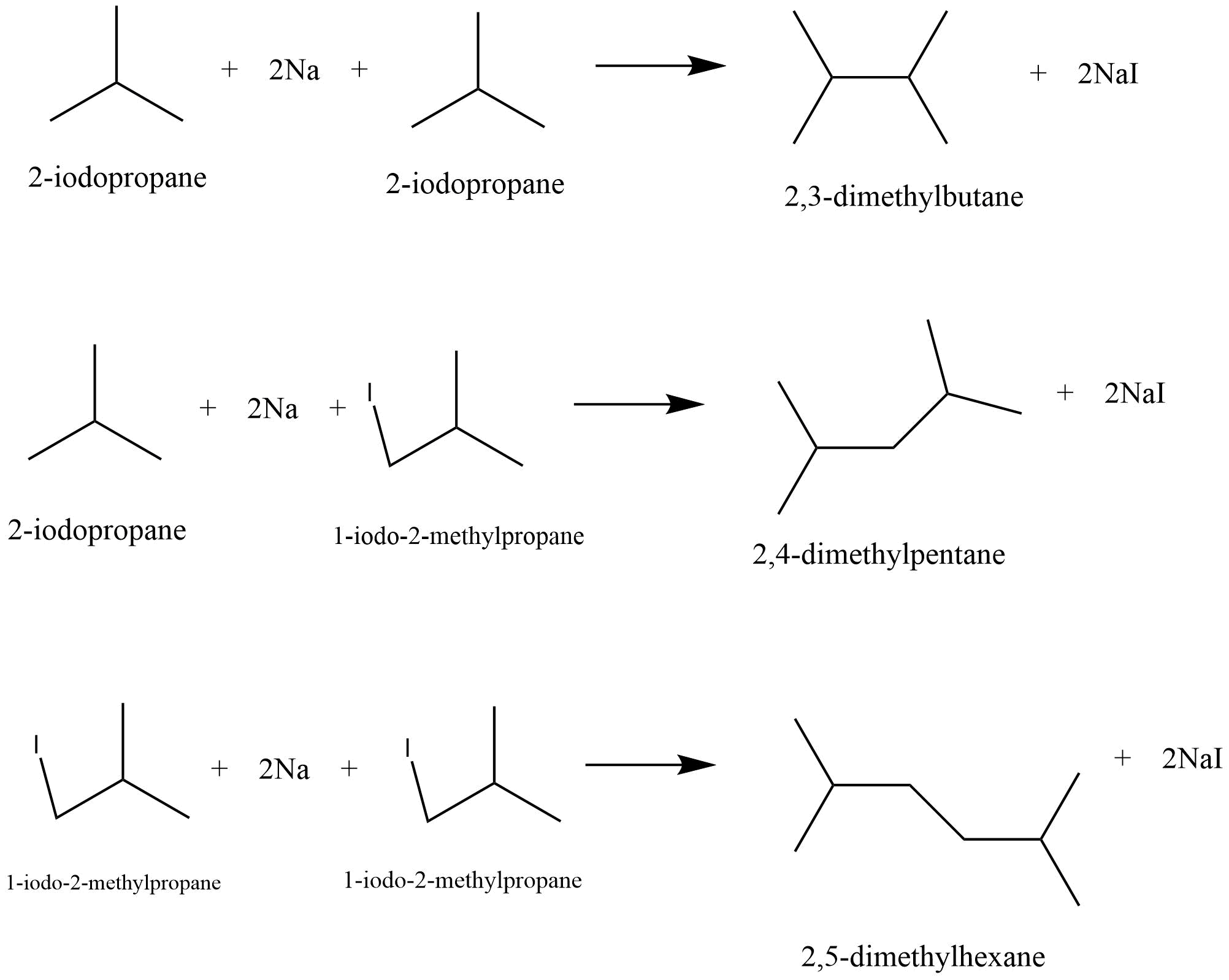
Preparation of alkanes
Properties and conformations of alkanes
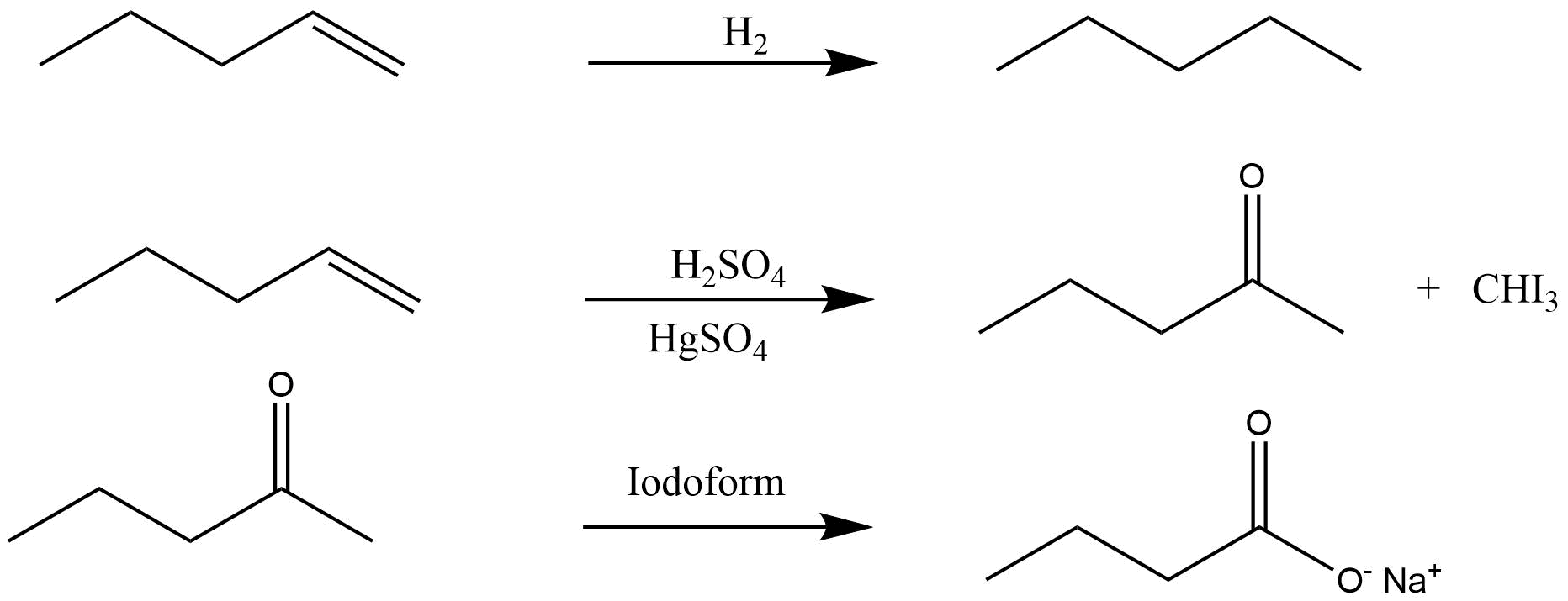
Alkenes
Structure Of Double Bond present in Alkenes
Nomenclature and isomerism of alkenes
Preparation and properties of it
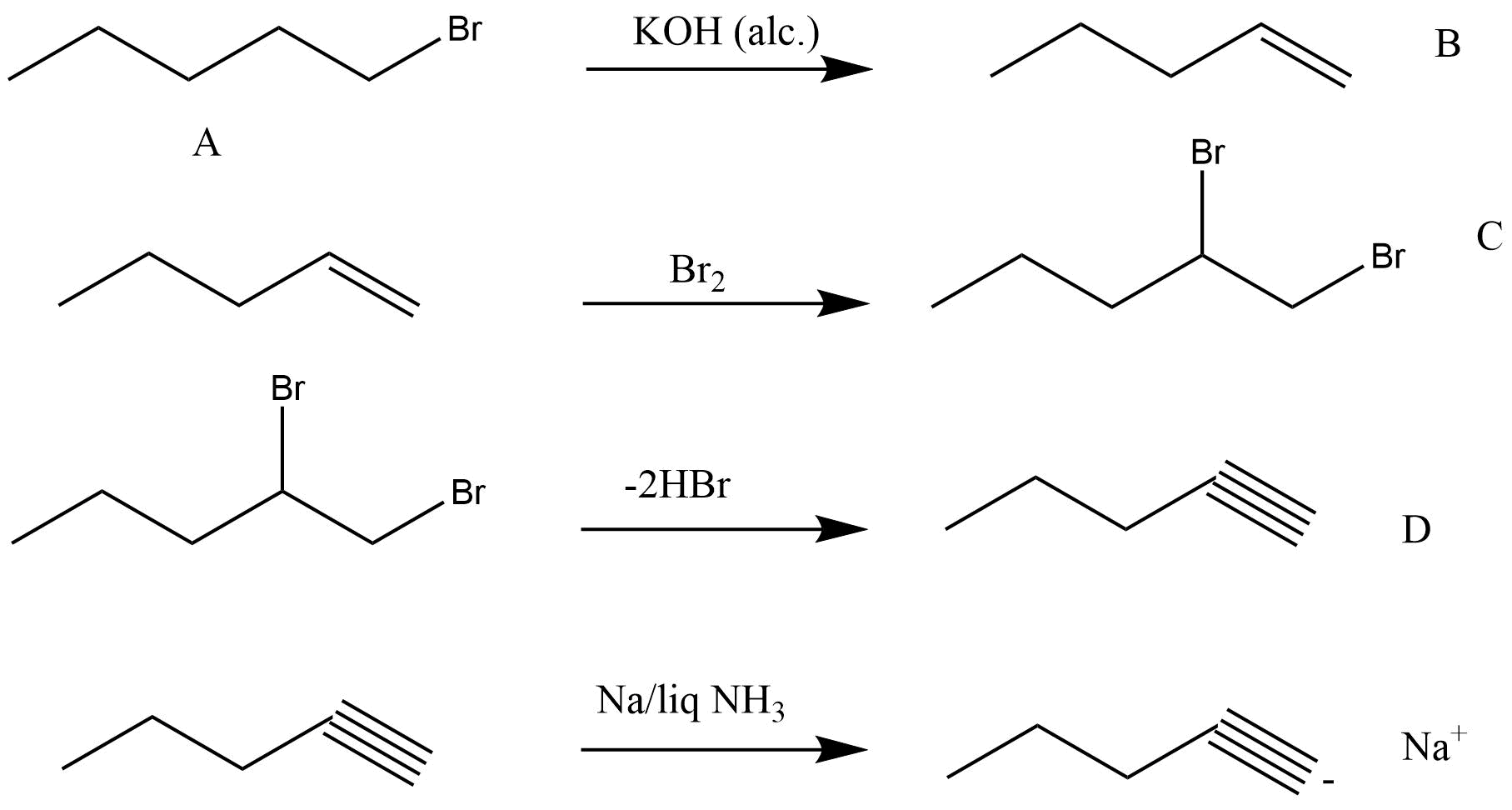
Alkynes
Nomenclature And Isomerism of alkynes
Structure Of Triple Bond present in Alkynes
Preparation and properties of it
Aromatic Hydrocarbon
Nomenclature And Isomerism of Aromatic hydrocarbons
Structure Of Benzene
Aromaticity in hydrocarbons
Preparation and Properties Of Benzene
Carcinogenicity And Toxicity of various hydrocarbons.
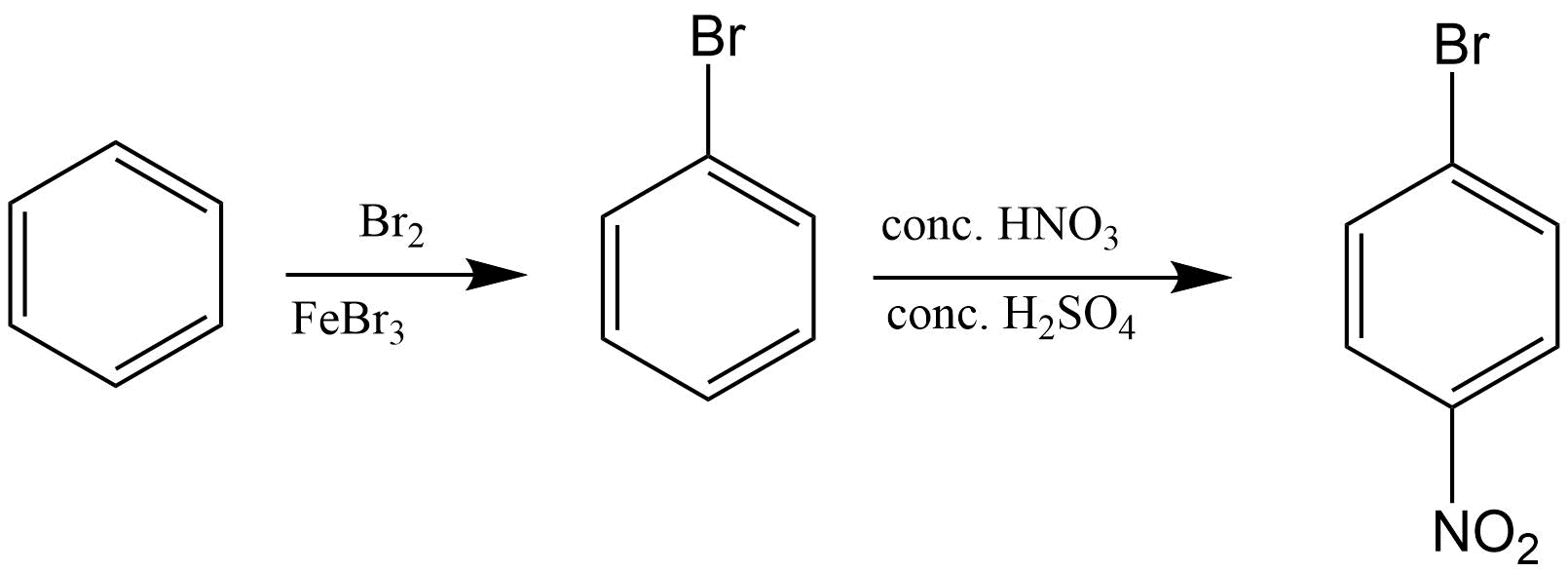
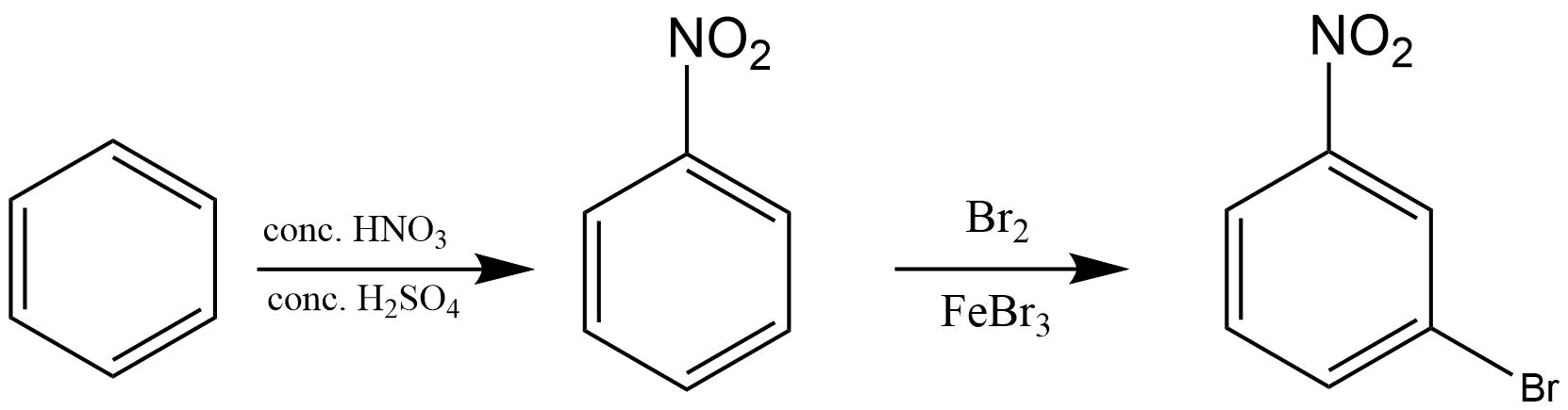
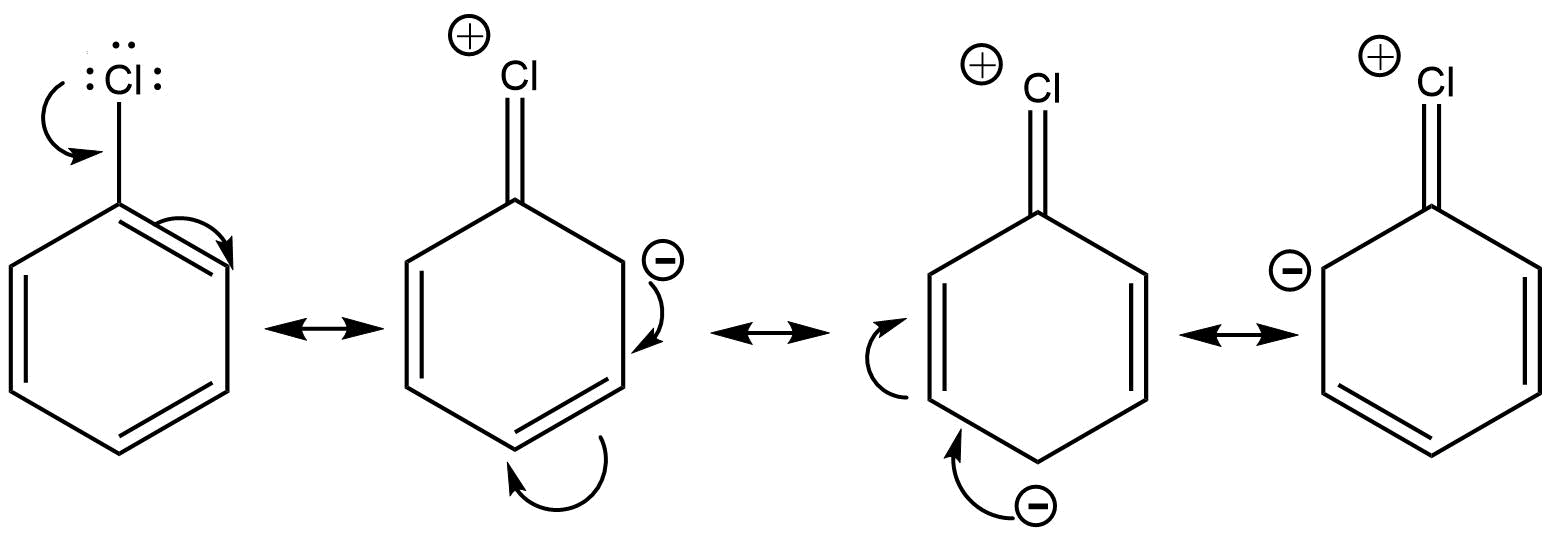
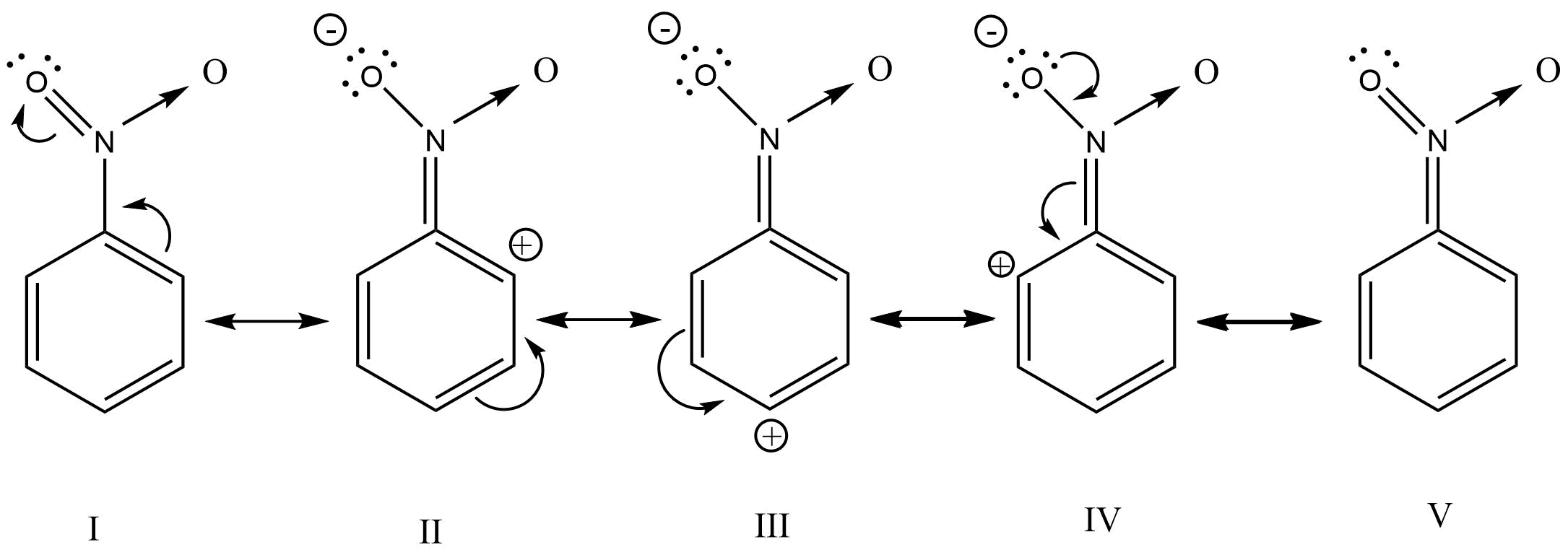
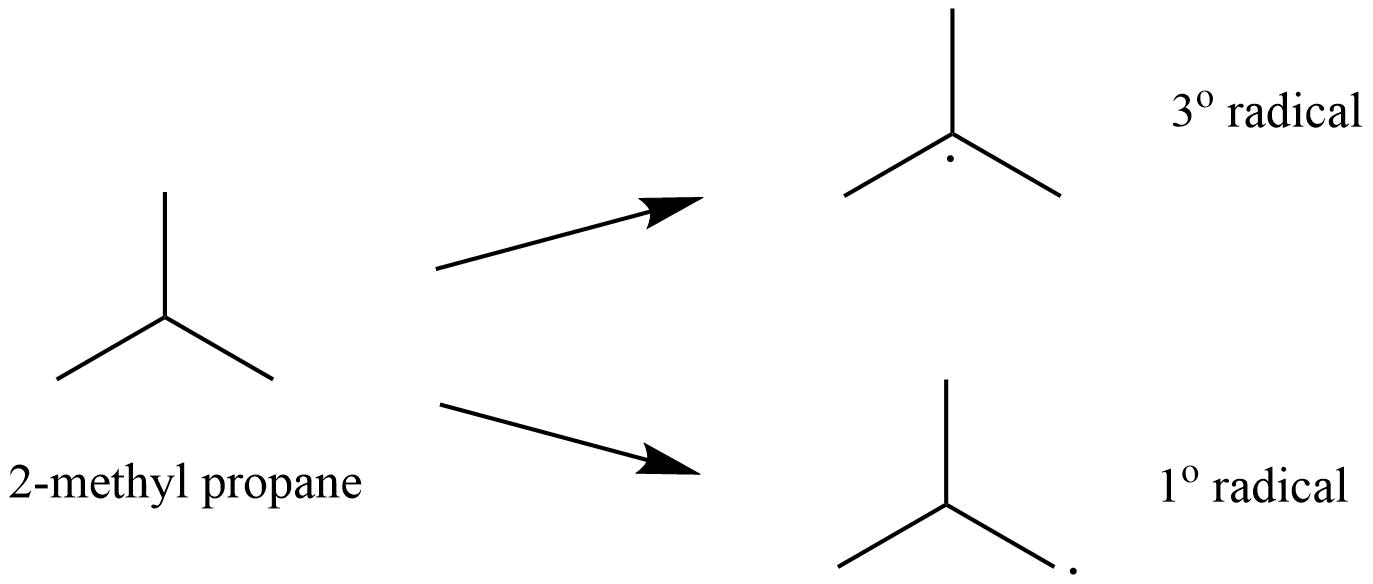
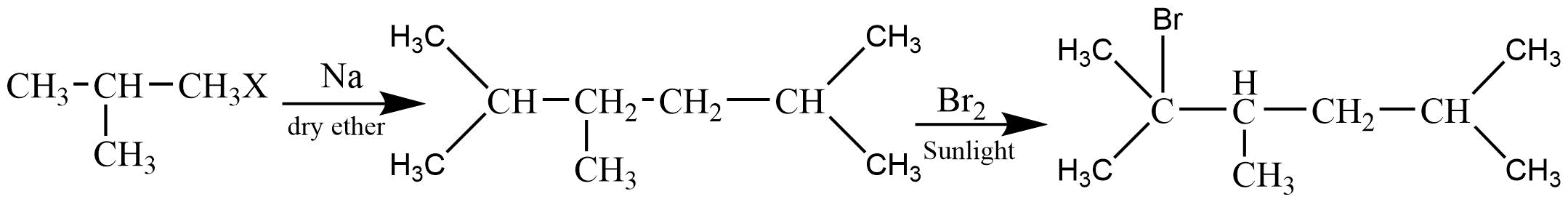
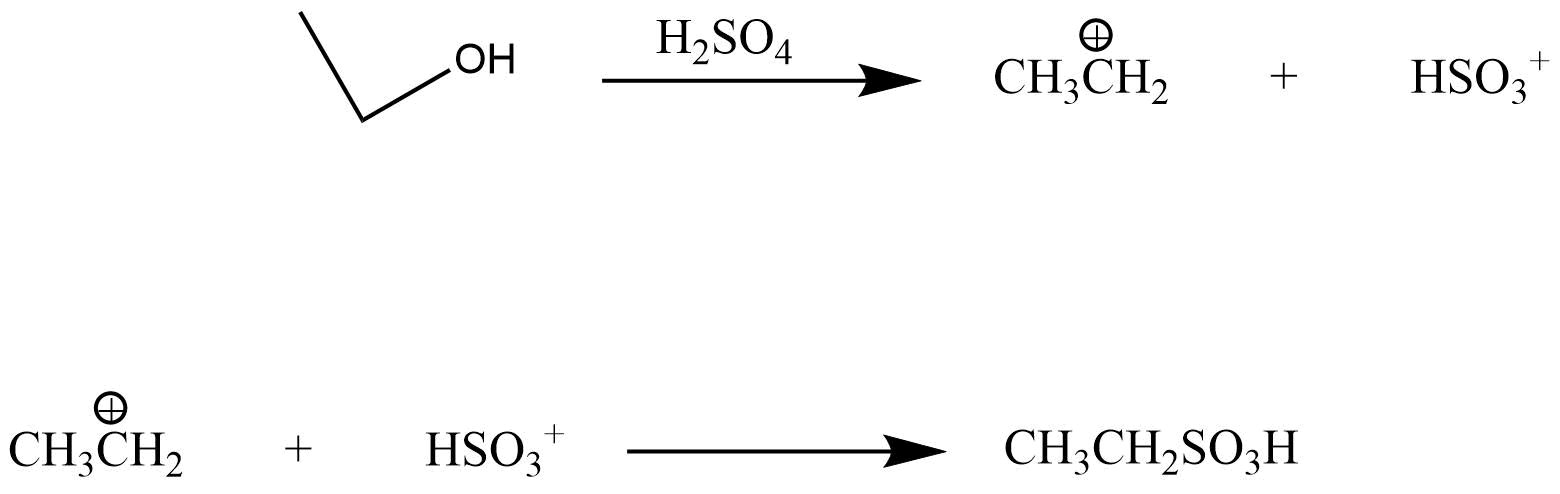
5. What is meant by halogenations of Alkanes according to chapter 13 Hydrocarbon?
Halogenation is a type of substitution reaction in the replacement of one or more atoms (Commonly Hydrogen atoms) of alkanes by the corresponding number of Halogen atoms (Halogen are elements of group 17 of the periodic table). For example -
a. Chlorination of Alkanes: when the replacing halogen atom is of chlorine it is called Chlorination of alkanes. It is carried out by treating alkane with chlorine at the temperature of 523 - 773K or in the presence of sunlight.
CH4 + Cl2 ⟶ CH3CL + HCL
b. Bromination of Alkanes: Bromination of alkanes works similarly but with bromine, and at a relatively slower pace.