NCERT Exemplar for Class 11 Chemistry Chapter 11 - The p-Block Elements - Free PDF Download
Chapter 11 of Class 11 Chemistry, the d-Block Elements, revolves around the 13 to 18 groups of the Periodic Table . These 6 groups are part of d-Block Elements, which contain a total of 31 Elements. These Elements consist of both metals and nonmetals, all the Metalloids are also included in this Chapter. Students can download free PDFs of the solutions to all the NCERT exemplar questions in this Chapter from the official website of vedantu.
Free PDF download of NCERT Exemplar for Class 11 Chemistry Chapter 11 - The p-Block Elements solved by expert Chemistry teachers on vedantu.com as per NCERT (CBSE) Book guidelines. All Chapter 11 - The p-Block Elements Exercise questions with solutions to help you to revise the complete syllabus and score more marks in your Examinations.
Access NCERT Exemplar Solutions for Class 11 Chemistry Chapter 11- The p-Block Elements
Multiple Choice Questions (Type I)
1. The element which exists in liquid state for a wide range of temperature and can be used for measuring high temperature is
(A) B
(B) Al
(C) Ga
(D) In
Ans: (C)
Explanation:
Gallium generally exists as solid at room temperature but melts on slight heating (melting point: $20^{\circ} \mathrm{C}$ ).
Whereas the boiling point of Gallium is very high around $2400^{\circ} \mathrm{C}$. Gallium has large cohesive forces that hold its structure together and it is stable for a wide range of temperatures and can be used for measuring high temperatures.
2. Which of the following is a Lewis acid?
(A) $\mathrm{AlCl}_{3}$
(B) $\mathrm{MgCl}_{2}$
(C) $\mathrm{CaCl}_{2}$
(D) $\mathrm{BaCl}_{2}$
Ans: $(A)$
Explanation:
Lewis acids are the species in which the state is not complete and ready to accept electrons. Because Al is surrounded by 6 electrons in $\mathrm{AlCl}_{3}$ and all three $\mathrm{Cl}$ atoms are surrounded by 8 electrons, $\mathrm{AlCl} 3$ is an electron acceptor. It is a covalent compound.
3. The geometry of a complex species can be understood from the knowledge of type of hybridisation
of orbitals of central atom. The hybridisation, of orbitals of central atom in $\left[\mathrm{B}(\mathrm{OH})_{4}\right]^{-}$and the geometry of the complex are respectively
(A) $\mathrm{sp}^{3}$, tetrahedral
(B) $\mathrm{sp}^{3}$, square planar
(C) $\mathrm{sp}^{3} \mathrm{~d}^{2}$, octahedral
(D) dsp $^{2}$, square planar
Ans: A
Explanation:
Central atom in $\left[\mathrm{B}(\mathrm{OH})_{4}\right.$ no lone pair. Electronic conf In ground state: $1 \mathrm{~s}^{2} 2 \mathrm{~s}^{2} 2 \mathrm{p}_{\mathrm{x}}^{1} 2 \mathrm{p}_{\mathrm{y}}^{0} 2 \mathrm{p}_{\mathrm{z}}^{0}$
In excited state, One Electron from $2 s$ shifts to $2 p y$ orbital and the configuration becomes:
$1 s^{2} 2 s^{2} 2 p^{1} x^{2} p^{1} y^{2} p_{z}^{0}$
Now, one ${ }^{s}$ and three $p$ orbitals combined to give ${ }^{s p}{ }^{3}$ hybridisation and tetrahedral shape.
4. Which of the following oxides is acidic in nature?
(A) $\mathrm{B}_{2} \mathrm{O}_{3}$
(B) $\mathrm{Al}_{2} \mathrm{O}_{3}$
(C) $\mathrm{Ga}_{2} \mathrm{O}_{3}$
(D) $\operatorname{In}_{2} \mathrm{O}_{3}$
Ans: A
Explanation:
The $\mathrm{B}_{2} \mathrm{O}_{3}$ reacts with water to form boric acid hence it is an acidic oxide. As we move down the group electronegativity decreases and the tendency to donate electrons increases therefore basic character increases or acidic character decreases. So, from the given options, the most acidic oxide js $\mathrm{B}_{2} \mathrm{O}_{3} .$
5. The exhibition of highest coordination number depends on the availability of vacant orbitals in the central atom. Which of the following elements is not likely to act as central atom in $\mathrm{MF}_{6}{ }^{3-}$ ?
(A) B
(B) AI
(C) Ga
(D) In
Ans: A
Explanation:
Number of items/molecules bonded to the central atom is termed as coordination number. In the given $\mathrm{MF}_{6}^{3-}$
Coordination number of metal is six and boron can have topmost coordination number of four as it consists of $s$ and $p$ orbital only and lacks d-orbital. Therefore, boron cannot be compared in the form of $\mathrm{MF}_{6}^{3-}$
6. Boric acid is an acid because its molecule
(A) contains replaceable $\mathrm{H}^{+}$ion
(B) gives up a proton.
(C)accepts $\mathrm{OH}^{-}$from water releasing proton.
(D) combines with proton from water molecule.
Ans: (C)
Explanation:
Boric acid is Lewis acid, having six electrons in its valence shell. It combines with water, accepts electrons
from $\mathrm{OH}^{-}$of water molecule and complete it octet to 8 and releases $\mathrm{H}^{+}$
Reaction: $\mathrm{B}(\mathrm{OH})_{3}+\mathrm{OH}-\mathrm{H} \rightarrow\left[\mathrm{B}(\mathrm{OH})_{4}\right]^{-}+\mathrm{H}^{+}$
7. Catenation, i.e. linking of similar atoms depends on size and electronic configuration of atoms. The tendency of catenation in Group 14 elements follows the order
(A) $\mathrm{C}>\mathrm{Si}>\mathrm{Ge}>\mathrm{Sn}$
(B) $\mathrm{C}>>\mathrm{Si}>\mathrm{Ge} \approx \mathrm{Sn}$
(C) $\mathrm{Si}>\mathrm{C}>\mathrm{Sn}>\mathrm{Ge}$
(D) $\mathrm{Ge}>\mathrm{Sn}>\mathrm{Si}>\mathrm{C}$
Ans: B
Explanation:
In group 14, as we move down from $\mathrm{C}$ to $\mathrm{Sn}$, the size increases and electronegativity decreases. This results in the decrease in M-M bond energy. Hence the catenation property decreases.
Bond energy is the amount of energy required to fragment the atoms combined in a molecule are born into separate atoms. Carbon has maximum bond energy as compared to its other group elements. Therefore, shows maximum catenation property.
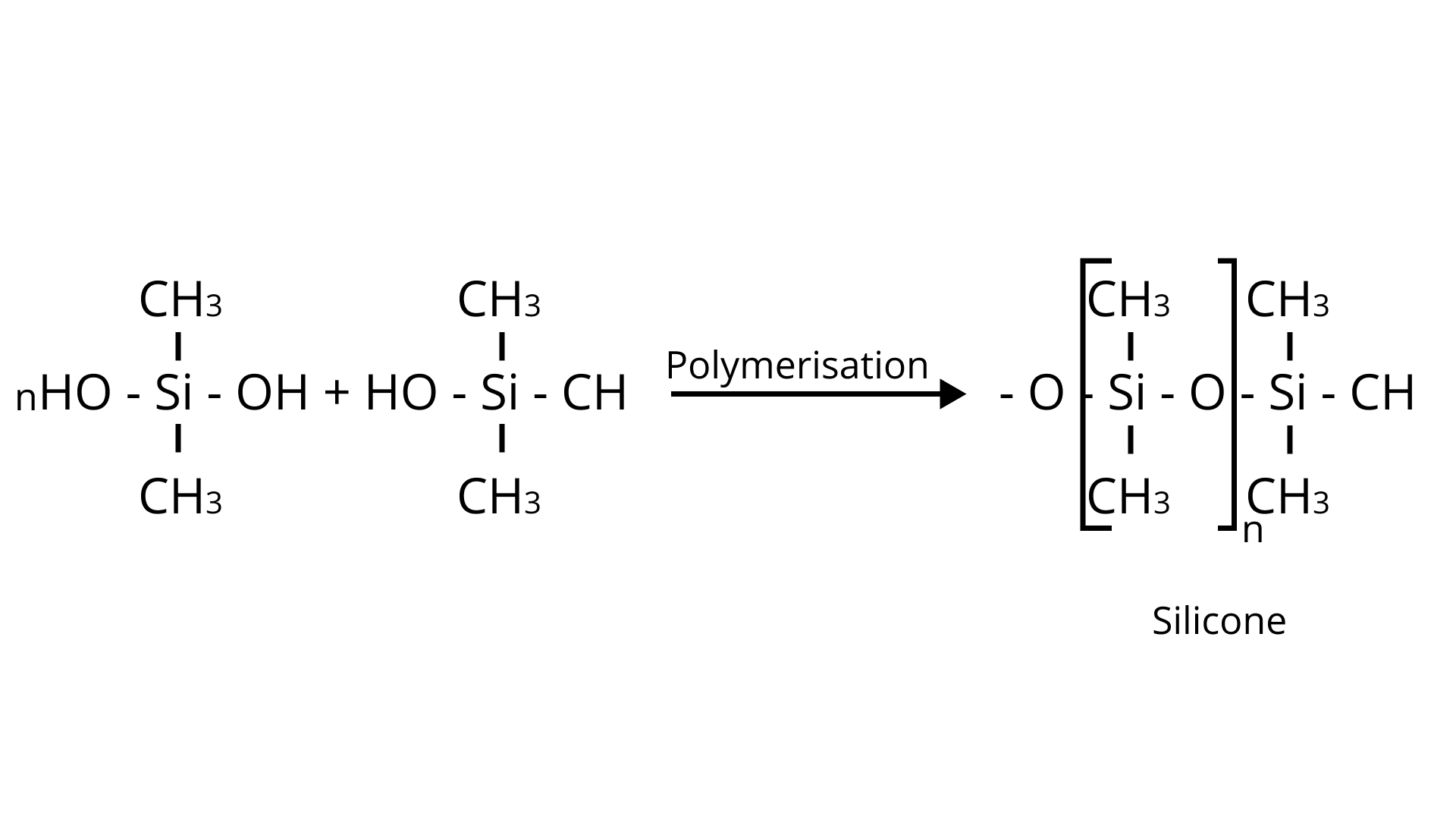
8. silicon has a strong tendency to form polymers like silicones. The chain length of silicone polymer can be controlled by adding
(A) $\mathrm{MeSiCl}_{3}$
(B) $\mathrm{Me}_{2} \mathrm{SiCl}_{2}$
(C) $\mathrm{Me}_{3} \mathrm{SiCl}$
(D) $\mathrm{Me}_{4} \mathrm{Si}$
Ans: C
Explanation:
$\mathrm{Me}_{3} \mathrm{SiCl}$ is a soleuless liquid That has a major application in the formation of silicones. On its addition in the process, it blocks the end and controls the chain length of the polymer.
Reaction:
9. Ionisation enthalpy $\left(\Delta_{\mathrm{t}} \mathrm{H}_{1} \mathrm{~kJ} \mathrm{~mol}^{-1}\right)$ for the elements of Group 13 follows the order.
(A) $\mathrm{B}>\mathrm{Al}>\mathrm{Ga}>\mathrm{In}>\mathrm{Tl}$
(B) $\mathrm{B}<\mathrm{Al}<\mathrm{Ga}<\mathrm{In}<\mathrm{T} 1$
(C) $\mathrm{B}<\mathrm{A} 1>\mathrm{Ga}<\mathrm{In}<\mathrm{T} 1$
(D) $\mathrm{B}>\mathrm{A} 1<\mathrm{Ga}>\mathrm{In}<\mathrm{T} 1$
Ans: D
Explanation:
In group 13, the standardised trend of decrease of ionization enthalpy is not monitored.
As we move from Boron to Aluminium the atomic size increases and jonjation enthalpy decreases but when we move ahead from Aluminum to Gallium, the screening effect of $3 \mathrm{~d}$ electrons comes into play. The poor shielding effect of the electrons lead to the increase in nuclear charge on the valence electrons and results in increase of jopisation enthalpy.
Moving from gallium to Indium, due to the shielding effect of $4 \mathrm{~d}$ electrons the ionization enthalpy decreases.
From Indium to thallium, $4 \mathrm{f}$ electrons come into action their poor shielding effect increases the effective nuclear charge on valence electrons hence the jonjation of Thallium energy further increases.
The decreasing order of ionization enthalpy for group-13 elements will be:
$\mathrm{B}>\mathrm{Tl}>\mathrm{Ga}>\mathrm{Al}>\mathrm{In}$
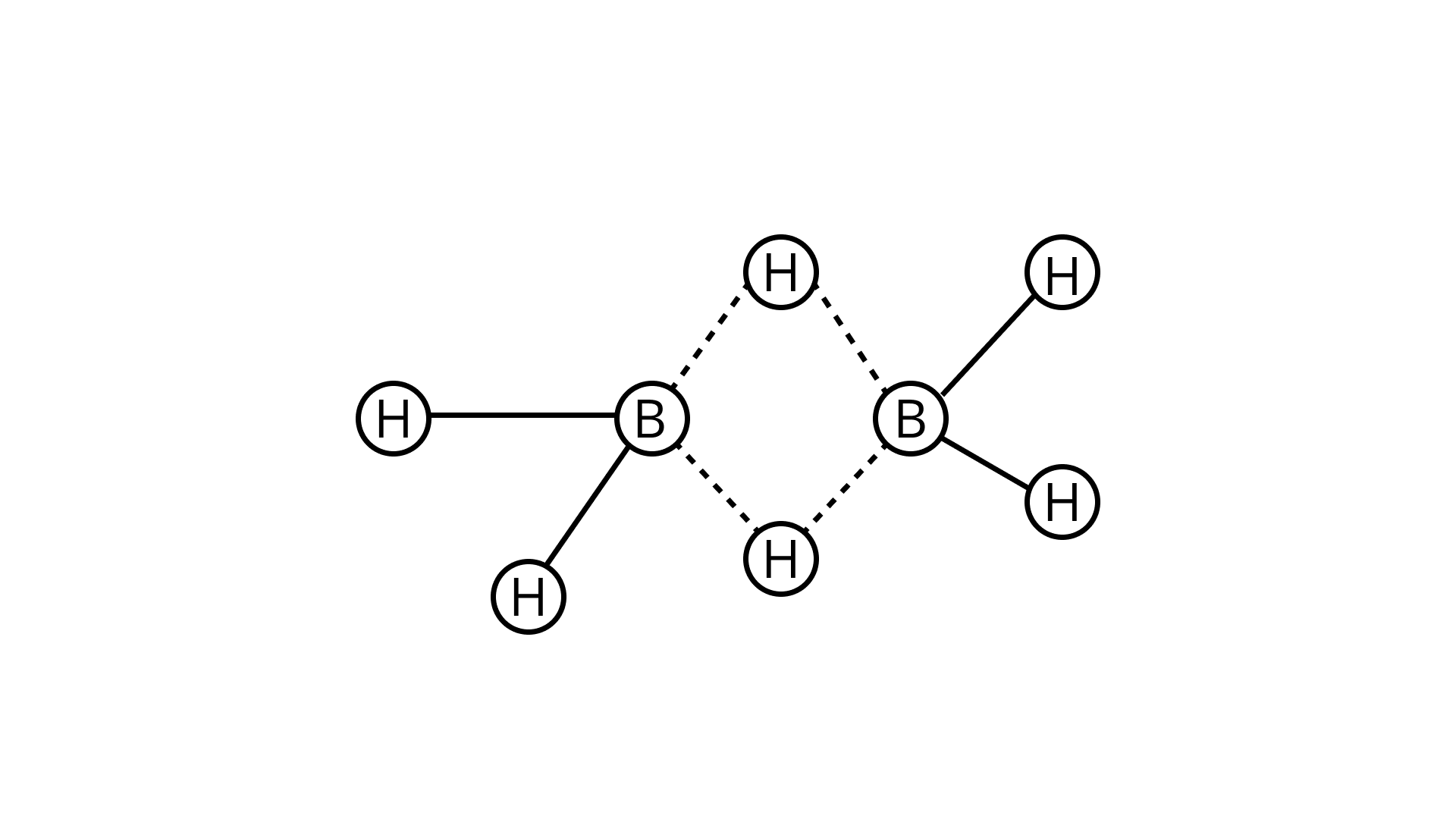
10. In the structure of Diborane
(a) all hydrogen atoms lie in one plane and boron atoms lie in a plane perpendicular to this plane.
(b) 2 boron atoms and 4 terminal hydrogen atoms lie in the same plane and 2 bridging hydrogen atoms lie in the perpendicular plane.
(c) 4 bridging hydrogen atoms and boron atoms lie in one plane and two terminal hydrogen atoms lie in a plane perpendicular to this plane.
(d) all the atoms are in the same plane.
Aps: (B)
Explanation:
The four terminal hydrogen atoms and two boron atoms are all in the same plane.
There are two bridging hydrogen atoms above and below this plane.
The four terminal B-H bonds are regular two-centre-two-electron bonds, whereas the two bridge (B-H-B) bonds are unique and can be described as three-centre-two-electron bonds, as shown in figure:
11. A compound $\mathrm{X}$, of boron reacts with $\mathrm{NH}_{3}$ on heating to give another compound $\mathrm{Y}$ which is called inorganic benzene. The compound X can be prepared by treating $\mathrm{BF}_{3}$ with lithium aluminium hydride. The compounds X and Y are represented by the formulas.
(A) $\mathrm{B}_{2} \mathrm{H}_{6}, \mathrm{~B}_{3} \mathrm{~N}_{3} \mathrm{H}_{6}$
(B) $\mathrm{B}_{2} 0_{3}, \mathrm{~B}_{3} \mathrm{~N}_{3} \mathrm{H}_{6}$
(C) $\mathrm{BF}_{3}, \mathrm{~B}_{3} \mathrm{~N}_{3} \mathrm{H}_{6}$
(D) $\mathrm{B}_{3} \mathrm{~N}_{3} \mathrm{H}_{6}, \mathrm{~B}_{2} \mathrm{H}_{6}$
Ans: A
Explanation:
A compound $\mathrm{X}$, of boron, reacts with $\mathrm{NH}_{3}$ on heating to give another compound $\mathrm{Y}$ which is called inorganic benzene.
$\underset{\mathrm{x}(\text { (Diborane) }}{3 \mathrm{H}_{2} \mathrm{H}_{6}}+6 \mathrm{NH}_{3} \rightarrow 3\left[\mathrm{BH}_{2}\left(\mathrm{NH}_{3}\right)_{2}\right]^{+}\left[\mathrm{BH}_{4}\right]^{-} \stackrel{\text { heat }}{\longrightarrow} \underset{\mathrm{Y} \text { (Borazole Inorganic Benzene) }}{2 \mathrm{~B}_{3} \mathrm{~N}_{3} \mathrm{H}_{6}}+12 \mathrm{H}_{2}$
$4 \mathrm{BF}_{3}+3 \mathrm{LiAlH}_{4} \rightarrow 2 \mathrm{~B}_{2} \mathrm{H}_{6}+3 \mathrm{LiF}+3 \mathrm{AlF}_{3}$
12. Quartz is extensively used as a piezoelectric material, it contains
(A) Pb
(B) Si
(C) Ti
(D) $\mathrm{Sn}$
Ans: B
Explanation:
Quartz is a crystalline form of silica that can be converted into other crystalline forms at high temperatures. It's commonly used as a piezoelectric material.
13. The most commonly used reducing agent is
(A) $\mathrm{AlCl}_{3}$
(B) $\mathrm{PbCl}_{2}$
(C) $\mathrm{SnCl}_{4}$
(D) $\mathrm{SnCl}_{2}$
Ans: (D)
Explanation:
Reducing agent are compound that donates electron to an electron recipient compound. Reducing agents oxidize themselves and reduce the other compounds. They easily lose electrons and increase their oxidation state; those electrons are accepted by electron needed compounds.
In $\mathrm{SnCl}_{2}, \mathrm{Sn}$ is in $+2$ oxidation state and can easily lose its two electrons and oxidize to $+4$ stable oxidation state.
Reaction: $\mathrm{SnCl}_{2}+2 \mathrm{Cl}^{-} \rightarrow \mathrm{SnCl}_{4}+2 \mathrm{e}^{-}$
14. Dry ice is
(A) Solid $\mathrm{NH}_{3}$
(B) Solid $\mathrm{SO}_{2}$
(C) solid $\mathrm{CO}_{2}$
(D) solid N $_{2}$
Ans: (C)
Explanation:
Solid $\mathrm{CO}_{2}$ is referred to as dry ice because it is used in laboratories to create an ice bath for organic reactions. It is made by rapidly cooling high-pressure $\mathrm{CO}_{2}$ gas.
15. Cement, the important building material is a mixture of oxides of several elements. Besides calcium, iron and sulfur, oxides of elements of which of the group(s) are present in the mixture?
(A) group 2
(B) groups 2,13 and 14
(C) groups 2 and 13
(D) groups 2 and 14
Ans: (B)
Explanation:
Cement is made by combining lime ( $\mathrm{CaO}$ ), clay with silica ( $\mathrm{SiO})$, and oxides of aluminum, magnesium, and iron.
Multiple Choice Questions (II)
16. The reason for small radius of Ga compared to $\mathrm{Al}$ is
(A) poor screening effect of $d$ and $f$ orbitals
(B) increase in nuclear charge
(C) presence of higher orbitals
(D) higher atomic number
Ans: (A) & (B)
Explanation:
The atomic radii decrease as one moves down the group from Al to Ga due to the shielding effect of electrons. As a result of this ineffective effect, the effective nuclear charge rises.
17. The linear shape of $\mathrm{CO}_{2}$ is due to
(A) ${s p^{3}}$ hybridisation of carbon
(B) $^{\mathrm{sp}}$ hybridisation of carbon
(C) $n-p \pi$ bonding between carbon and oxygen
(D) $^{\mathrm{sp}^{2}}$ hybridisation of carbon
Ans: (B) & (C)
Explanation:
Hybridisation of any atom in a molecule corresponds to the number or $\sigma$ bonds it is making covalently with other atoms.
In $\mathrm{CO}_{2}$, carbon is attached to two oxygen atoms through double bonds. Out of which two are $\sigma$ bonds and the rest two are $\pi$ bonds forming $\mathrm{I}-\mathrm{p} \pi$ bonding between carbon and oxygen.
The two $\sigma$ bonds correspond to the hybridization ${ }^{s p}$ and give a linear shape to $\mathrm{CO}_{2}$.
18. $\mathrm{Me}_{3} \mathrm{SiCl}$ is used during polvmerisation of grgane silicones because
(A) the chain length of orange silicone polymers can be controlled by adding $\mathrm{Me}_{3}$
(B) $\mathrm{Me}_{3} \mathrm{SiCl}$ blocks, the end terminal of silicone polymer.
(C) $\mathrm{Me}_{3} \mathrm{SiCl}$ improves the quality and yield of the polymer.
(D) $\mathrm{Me}_{3} \mathrm{SiCl}$ acts as a catalyst during polymerization.
Ans: (A) & (B)
Explanation:
$\mathrm{Me}_{3} \mathrm{SiCl}$ is a soleuless liquid and is stable in anhydrous condition. When added in the process of polymerization, it blocks the end terminal of the silicone polymer and determines its chain length.
19. Which of the following statements are correct?
(A) Fullerenes have dangling bonds.
(B) Fullerenes are cage-like molecules.
(C) Graphite is thermodynamically most stable allotrope of carbon.
(D) Graphite is slippery and hard and therefore used as a dry lubricant in machines.
Ans: (B) & (C)
Explanation:
(B) Fullerene is an allotrope of carbon consisting of 60 carbon atoms bonded together through single and double bonds in such a manner that they form a hollow sphere like shape or a cage-like structure.
(C) In graphite, each carbon atom has one free/ delocalised electron that causes strong attraction between carbon atoms providing more stability to the overall structure of graphite.
20. Which of the following statements are correct? Answer on the basis of figure.
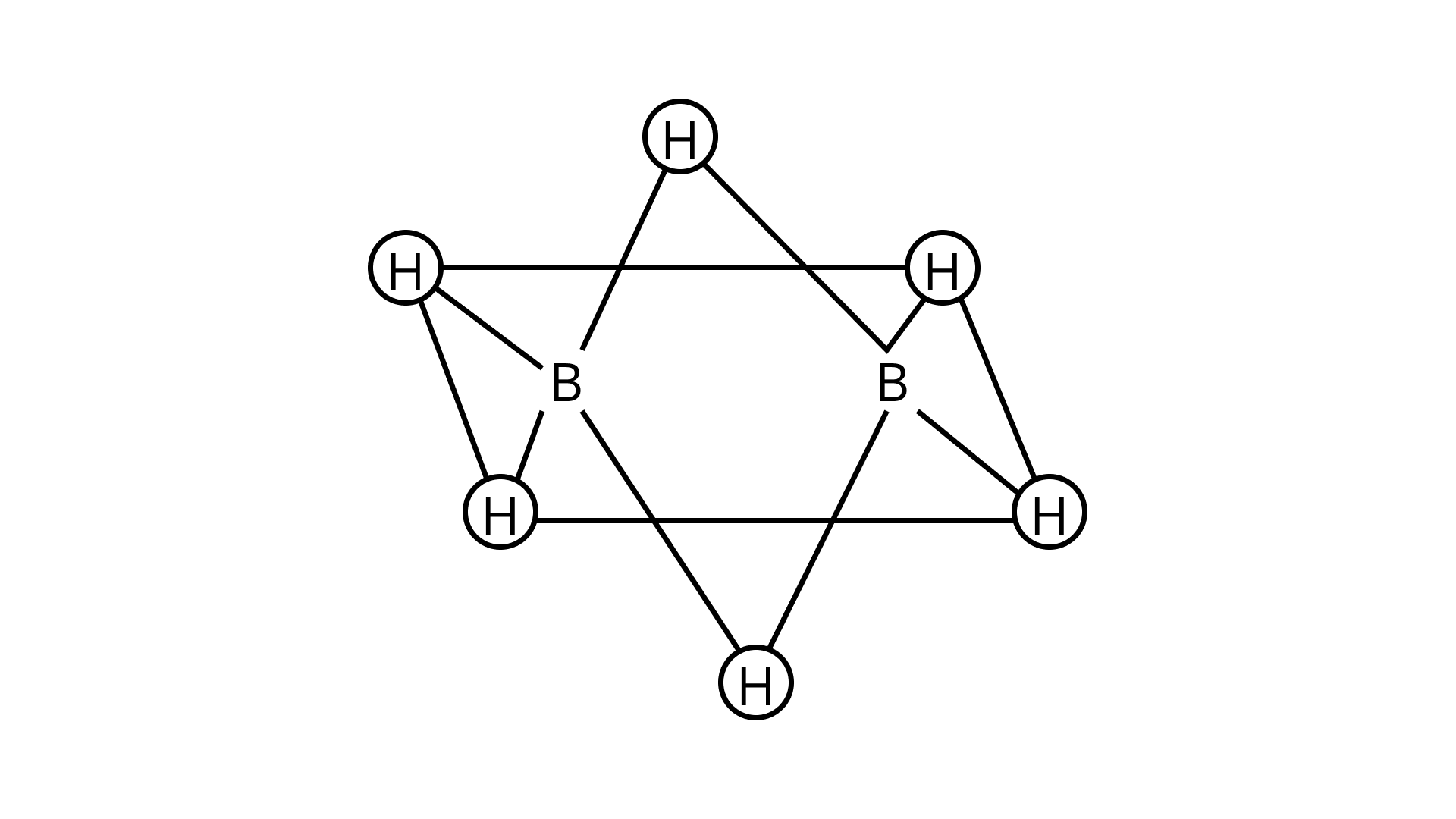
(A) The two bridged hydrogen atoms and the two boron atoms lie in one plane.
(B) Out of six B - H, bonds two bonds can be described in terms of 3 centre 2-electron bonds.
(C) Out of six B- H bonds four B- H bonds can be described in terms of 3 centre 2 electron bonds.
(D) The four terminal B - H bonds are two centre-two electron regular bonds.
Ans: (A), (B) & (D)
Explanation: Two hydrogens forming bridge in $\mathrm{B}_{2} \mathrm{H}_{6}$ are peculiar in bonding and can be termed as
3 -centered-2-electron bond or banana bond. $1 \mathrm{~s}_{\text {grbital }}$ of each hydrogen overlaps with the hybrid
orbital of one of the boron then delocalising the $2 \mathrm{e}^{-}$over three atoms making 3 -centered-2-electron bond.
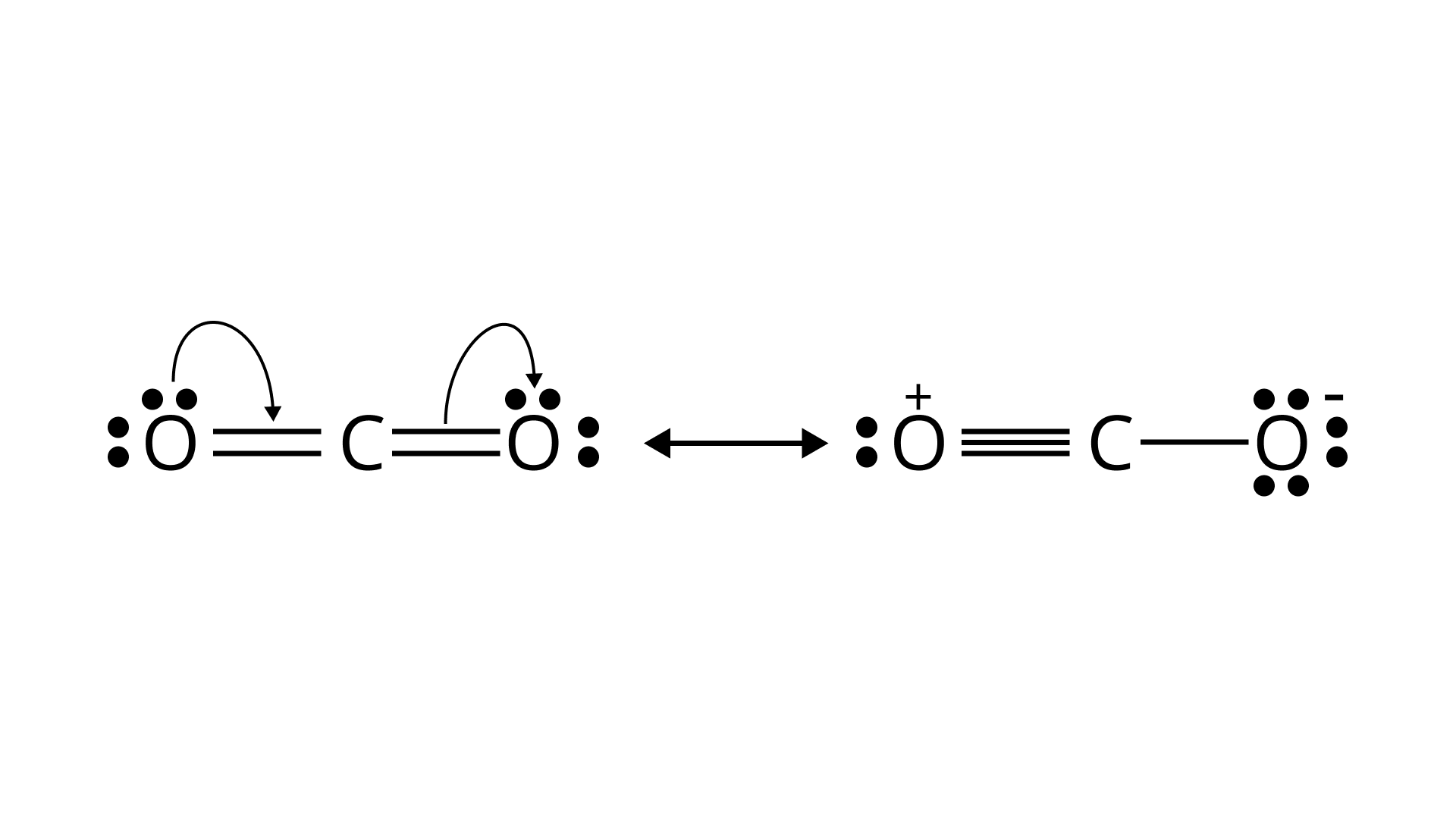
21. Identify the correct resonance structures of carbon dioxide from the ones given below:
(A) $\mathrm{O}-\mathrm{C} \equiv \mathrm{O}$
(B) $\mathrm{O}=\mathrm{C}=\mathrm{O}$
(C) ${ }^{-} \mathrm{O} \equiv \mathrm{C}-\mathrm{O}^{+}$
(D) ${ }^{-} \mathrm{O}-\mathrm{C} \equiv \mathrm{O}^{+}$
Ans: (B) & (D)
Explanation:
The following is the resonance structure of $\mathrm{CO}_{2}$.
Short Answer Type
22. Draw the structures of $\mathrm{BCl}_{3} \cdot \mathrm{NH}_{3}$ and $\mathrm{AlCl}_{3}$ (dimer).
Ans:
The central $\mathrm{B}$ atom in $\mathrm{BCl}_{3}$ has six electrons in its valence shell. $\mathrm{As}$ a result, it is an electron-deficient molecule in need of two more electrons to complete its octet. To put it another way, $\mathrm{BCl}_{3}$ acts as a Lewis acid. $\mathrm{NH} 3$ on the other hand, has a lone pair of electrons that it can easily donate. $\mathrm{As}$ as a result, $\mathrm{NH}_{3}$ serves as a Lewis base. As shown below, the Lewis acid $\left(\mathrm{BCl}_{3}\right)$ and Lewis base $\left(\mathrm{NH}_{3}\right)$ combine to form an adduct:
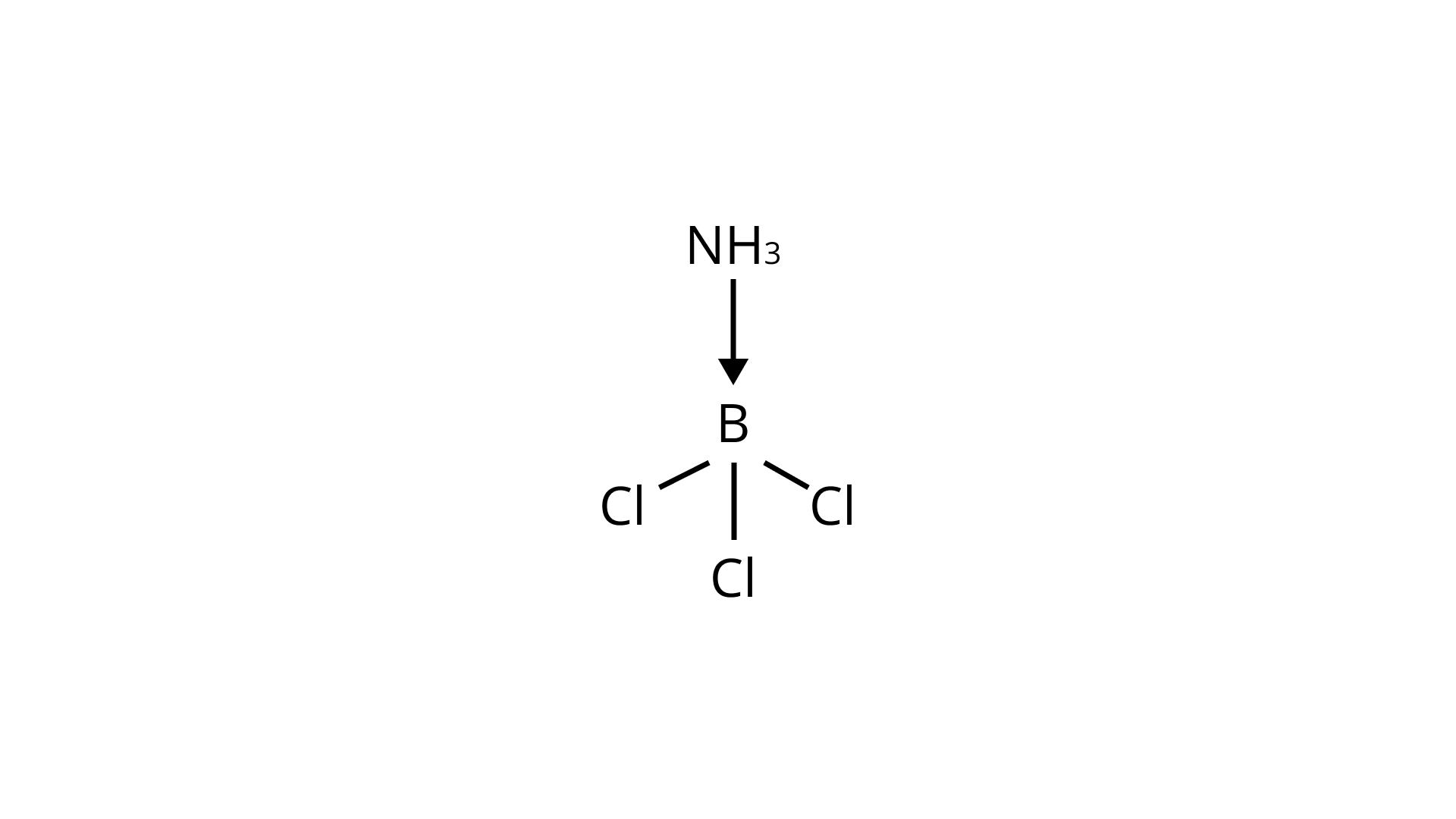
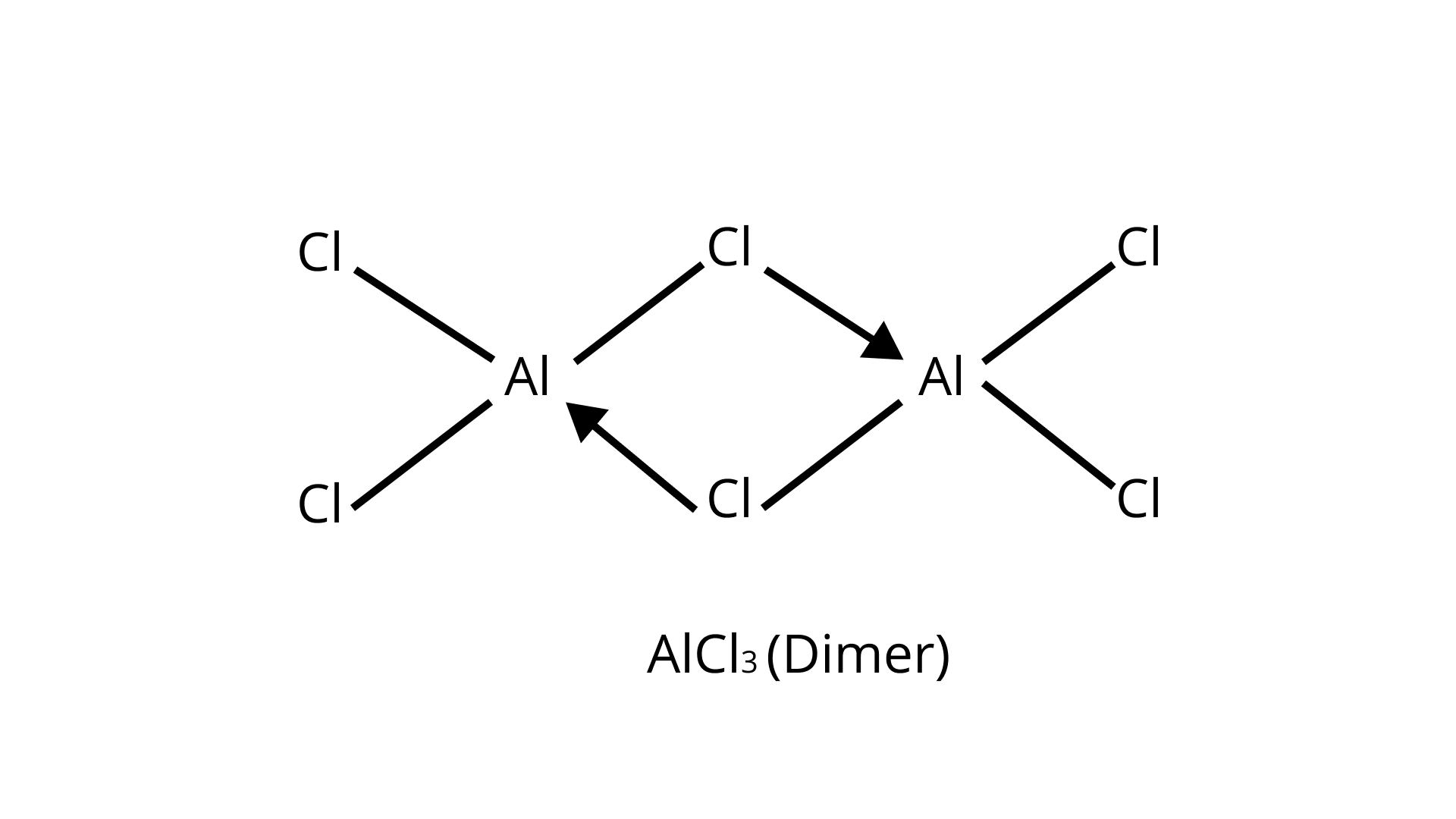
The valence shell of AlCl3 contains six electrons. As a result, it is an electron-deficient molecule that requires two additional electrons to complete its octet.
Chlorine, on the other hand, has three lone electron pairs. As a result, to complete its octet, the central Al atom of one molecule accepts a lone pair of electrons from the Cl atom of the other molecule, resulting in the formation of a dimeric structure, as shown below.
23. Explain the nature of boric acid as a Lewis acid in water.
Ans: $\mathrm{H}_{3} \mathrm{BO}_{3}$ or $\mathrm{B}(\mathrm{OH})_{3}$ is an electron deficient compound or Lewis acid which easily accepts electron from $\mathrm{OH}$ of water and releases its proton hence it is a Monobasic acid.
Reaction:
$\mathrm{B}(\mathrm{OH})_{3}+2 \mathrm{H}_{2} \mathrm{O} \rightarrow\left[\mathrm{B}(\mathrm{OH})_{4}\right]^{-}+\mathrm{H}_{3} \mathrm{O}^{+}$
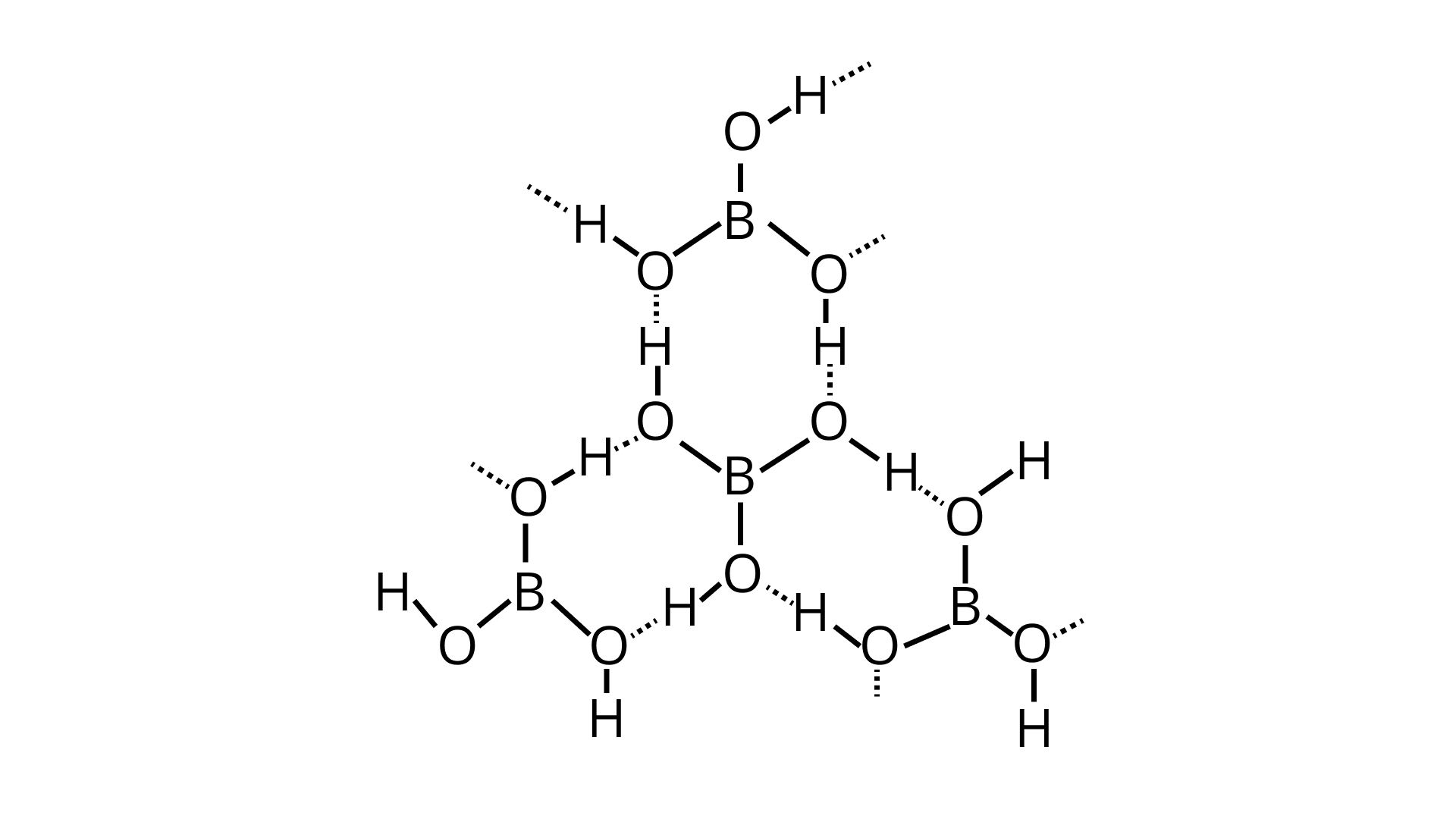
24. Draw the structure of boric acid showing hydrogen bonding. Which species is present in water? What is the hybridisation of boron in this species?
Ans:
$\mathrm{H}_{3} \mathrm{BO}_{3}$
Boric acid forms a hexagon and rings through hydrogen bonding and has a layer-like structure.
Boric acid is present in water as $\left[\mathrm{B}(\mathrm{OH})_{4}\right]^{-} . \mathrm{H}_{3} \mathrm{BO}_{3}$ electron from the $\mathrm{OH}$ of water and forms the complex BOH for negative for $s p^{3}$ and is present in SP3 hybridisation.
Reaction:
$\mathrm{B}(\mathrm{OH})_{3}+2 \mathrm{H}_{2} \mathrm{O} \rightarrow\left[\mathrm{B}(\mathrm{OH})_{4}\right]^{-}+\mathrm{H}_{3} \mathrm{O}^{+}$
25. Explain why the following compounds behave as Lewis acids?
(j) $\mathrm{BCl}_{3}$
(ii) $\mathrm{AlCl}_{3}$
Ans:
Both $\mathrm{BCl}_{3}$ and $\mathrm{AlCl}_{3}$ are electron deficient compounds that are central atom boron and aluminium have incomplete Octet. In each compound, a metal atom is surrounded by six electrons of three covalent bonds with 3 chlorine atoms.
Each chlorine atom has a complete Octet of eight electrons. The electron deficient compounds act as Lewis acid and readily accept two electrons to complete their octet.
26. Give reasons for the following:
(A) $\mathrm{CCl}_{4}$ is immiscible in water, whereas $\mathrm{SiCl}_{4}$ is easily hydrolysed.
(B) Carbon has a strong tendency for catenation compared to silicon.
Ans:
(A) sarbon in $\mathrm{CCl}_{4}$ does not have a vacant d-orbital to accommodate the electrons from $\mathrm{OH}$ of water molecules. Also $\mathrm{CCl}_{4}$ is nonpolar covalent compounds whereas $\mathrm{H}_{2} \mathrm{O}$ is polar. So, no strong interaction occurs between them. Hence $\mathrm{CCl}_{4}$ is miscible in water.
Whereas in $\mathrm{SiCl}_{4}$, silicon has bigger size than carbon and have $\mathrm{d}$-orbitals for accommodation of electrons donated by $\mathrm{OH}$ of water in the process of hydroxylation. This leads to a strong interaction and silicon acid Is formed as a product. $\mathrm{SiCl}_{4}$ is completely miscible in water.
(B) As we move from carbon to silicon, the size increases and electronegativity decreases. This results in the decrease in M-M bond energy. Hence the catenation property decreases.
Bond energy is the amount of energy required to fragment the atoms combined in a molecule are born into separate atoms. Carbon has maximum bond energy as compared to silicon. Therefore, shows maximum catenation property.
27. Explain the following:
(A) $\mathrm{CO}_{2}$ is a gas whereas $\mathrm{SiO}_{2}$ is a solid.
(B) Silicon forms $\mathrm{SiF}_{6}^{2-}$ ion whereas corresponding fluoro-compound of carbon is not known.
Ans:
(A) Carbon has small size and large electronegativity, it forms strong $\mathrm{n}-\mathrm{p} \pi$ bonding with two oxygen atoms forming a separate $\mathrm{CO}_{2}$ molecule.
While in $\mathrm{SiO}_{2}$ silicon is larger in size with comparatively less electronegativity than carbon it shows no tendency to form $\mathrm{n}-\mathrm{p} \pi$ bonding rather forms Single covalent bond with oxygen. Thus, $\mathrm{SiO}_{2}$ possess $3 \mathrm{D}$ network-like structure in which each Silicon is bonded to 4 oxygen atoms.
(B) Carbon is smaller in size and lacks d-orbitals hence can have a maximum coordination number of four and $s p^{3}$ hybridisation only.
Whereas Silicon has d-orbital, it can amplify its hybridisation up to $\mathrm{sp}^{3} d^{2}$ (CN=6) and can complete its Octet. Therefore, Silicon forms $\mathrm{SiF}_{6}^{2-}$.
whereas the corresponding flugre-compound of carbon is not known.
28. The $+1$ oxidation state in group 13 and $+2$ oxidation state in group 14 becomes more and more stable with increasing atomic number. Explain.
Ans: As we move down the group in group 13 and 14 the participation of s-electrons in bond formation decreases the primary reason behind this is the inert pair effect.
In this the p-electrons take part in bond formation and more energy is required to unpack the valence electrons to make them participate in bonding. Due to this the lower oxidation state of elements becomes stable done the hire oxidation state. As for group $13,+1$ oxidation state is more stable than $+3$ and for group $14,+2$ oxidation state is more stable than $+4 .$
29. Carbon and silicon both belong to the group 14, but in spite of the stoichiometric similarity, the dioxides (i.e., carbon dioxide and silicon dioxide) differ in their structures. Comment.
Ans: As compared to carbon, silicon is bigger in size and is less electronegative. It shows resistance in forming $\mathrm{p}-\mathrm{p}$ multiple bonding which is easily done by carbon. Thus, $\mathrm{SiO}_{2}$ is a $3-\mathrm{D}$ network where each silicon is linked covalently to 4 oxygen atoms while in $\mathrm{CO}_{2}$, Carbon is linked with two oxygen atoms with double bond in a linear manner.
30. If a trivalent atom replaces a few silicon atoms in three dimensional network of silicon dioxide, what would be the type of charge on overall structure?
Ans: When a trivalent atom is added to the crystal of $\mathrm{SiO}_{2}$, it substitutes silicon atoms which result in generation of holes. These holes make the crystal conductor of electricity. The overall Crystal is electrically neutral and is called the $p$-type conductor.
31. When $\mathrm{BCl}_{3}$ is treated with water, it hydrolyses and forms $\left[\mathrm{B}(\mathrm{OH})_{4}\right]^{-}$only whereas $\mathrm{AlCl}_{3}$ in acidified aqueous solution forms $\left[\mathrm{Al}\left(\mathrm{H}_{2} 0\right)_{6}\right]^{3+}$
Explain what is the hybridisation of boron and aluminium in these species?
Ans:
$\mathrm{BCl}_{3}+3 \mathrm{H}_{2} \mathrm{O} \rightarrow \mathrm{B}(\mathrm{OH})_{3}+3 \mathrm{HCl}$ $\mathrm{B}(\mathrm{OH})_{3}+2 \mathrm{H}_{2} \mathrm{O} \rightarrow\left[\mathrm{B}(\mathrm{OH})_{4}\right]^{-}+\mathrm{H}_{3} \mathrm{O}^{+}$ Boron in $\mathrm{B}(\mathrm{OH})_{3}$ has configucation $^{2} 2 \mathrm{~s}^{2} 2 \mathrm{p}_{x}^{1} \times 2 \mathrm{p}_{y}^{0} 2 \mathrm{p}^{0}_{z}$.
And in $\left[\mathrm{B}(\mathrm{OH})_{4}\right]^{-}$, one electron shifts from $2 \mathrm{~s}$ to $2 \mathrm{p}^{0} \mathrm{y}$. So the configuration becomes:
$1 \mathrm{~s}^{2} 2 \mathrm{~s}^{2} 2 \mathrm{p}_{\mathrm{x}}^{1} 2 \mathrm{p}^{1} \mathrm{y} 2 \mathrm{p}_{\mathrm{z}}^{0} .$ As, one $^{2 \mathrm{~s}}$ and three $^{2 \mathrm{p}}$ orbitals are involved giving the hybridjation $^{\mathrm{sp}^{3}}$. In case of aluminump, reaction involved is:
$\mathrm{AlCl}_{3}+6 \mathrm{H}_{2} \mathrm{O} \stackrel{H C l}{\longrightarrow}\left[\mathrm{Al}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{3+}$
In $\mathrm{Al}^{3+}$, the electrons $3 \mathrm{~s}, 3 \mathrm{p} \& 3 \mathrm{~d}$ orbitals are vacant. So the six electrons from water are accommodated in one s-orbital, three p-orbitals and two d-orbitals. Thus giving the hybridization sp $\mathrm{d}^{2}$.
32. Aluminium dissolves in mineral acids and aqueous alkalis and thus shows amphoteric character, a piece of aluminium foil is treated with dilute hydrochloric acid or dilute sodium hydroxide solution in a test tube and on bringing a burning matchstick near the mouth of the test tube, a pop sound indicates the evolution of hydrogen gas. The same activity when performed with concentrated nitric acid, reaction doesn't proceed. Explain the reason.
Ans: Nature of aluminium is amphoteric which means it can react with acid as well as base to produce salt and hydrogen gas as products. Hydrogen gas produces a popping sound on burning.
Reaction:
$2 \mathrm{Al}+6 \mathrm{HCl} \longrightarrow 2 \mathrm{AlCl}_{3}+3 \mathrm{H}_{2}$
${2 \mathrm{Al}+2 \mathrm{NaOH}+2 \mathrm{H}_{2} \mathrm{O} \rightarrow}{2 \mathrm{NaAlO}_{2}+3 \mathrm{H}_{2}}$
33. Explain the following:
A. Gallium has higher ionization enthalpy than aluminium.
B. Boron does not exist as $\mathrm{B}^{3+}\left(-\mathrm{R}_{2} \mathrm{SiO}-\right)$
C. Aluminium forms $\left[\mathrm{A} 1 \mathrm{~F}_{6}\right]^{3-}$ ion but boron does not form $\left[\mathrm{BF}_{6}\right]^{3-}$
D. $\mathrm{PbX}_{2}$ is more stable than $\mathrm{PbX}_{4}$.
E. $\mathrm{Pb}^{4+}$ acts as an oxidising agent but $\mathrm{Sn}^{2+}$ acts as a reducing agent.
F. Electron gain enthalpy of chlorine is more negative as compared to fluorine.
G. $\mathrm{TI}\left(\mathrm{NO}_{3}\right)_{3}$ acts as an oxidizing agent.
H. Carbon shows catenation property but lead does not.
I. $\mathrm{BF}_{3}$ does not hydrolyse,
J. Why does the element silicon not form a graphite like structure whereas carbon does.
Ans:
A. As we move from Aluminium to Gallium, the screening effect of $3 \mathrm{~d}$ electrons comes into play. The poor shielding effect of the electrons lead to the increase in nuclear charge on the valence electrons and results in increase of jonjatign enthalpy.
B. As boron is smaller in size and the sum of its first three ionization enthalpies i.e. $\Delta \mathrm{H}_{1}+\Delta \mathrm{H}_{2}+\Delta \mathrm{H}_{3}$ is very large so boron does not allow to lose its all three valence electrons and exist as $+3$ ion rather it shares electrons to make covalent bonds.
C. As compared to boron, aluminium is larger in size and has vacant d-orbital through which it can expand its coordination number from $+4$ to $+6$. Can easily acquire the $s p^{3} d^{2}$ hybridisation State. On the other hand, boron is smaller in size with no $d$-orbitals and can expand its coordination number maximum up to $+4$ so boron can maximum the form $\mathrm{BF}_{4}{ }^{-}$but not $\mathrm{BF}_{6}{ }^{2-}$.
D. Lead is extra stable in its $+2$ oxidation state than in $+4$ oxidation state due to inert pair effect.
E. Due to the inert Pair effect, lead is more stable in its $+2$ oxidation state than in $+4$. So if it is present in $+4$ oxidation state in any compound it can readily reduce itself by gaining two electrons. Thus acting as an oxidising agent.
Reaction: $\mathrm{Pb}^{4+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Pb}^{2+}$
While for $\operatorname{Sn}^{2+},+4$ oxidation state is extra stable than $+2$ oxidation state so in any compound where acid is present in $+2$ oxidation state it loses two more electron and gxidises itself to $+4$ oxidation state. Thus acting as a reducing agent.
Reaction: $\mathrm{Sn}^{2+} \rightarrow \mathrm{Sn}^{4+}+2 \mathrm{e}^{-}$
F. Electronic configuration of $\mathrm{F} \& \mathrm{Cl}_{\text {are: }}$
$\mathrm{F}(Z=9)=1 \mathrm{~s}^{2} 2 \mathrm{~s}^{2} 2 \mathrm{p}^{5}$
$\mathrm{Cl}(Z=17)=1 \mathrm{~s}^{2} 2 \mathrm{~s}^{2} 2 \mathrm{p}^{6} 3 \mathrm{~s}^{2} 3 \mathrm{p}^{5}$
In fluorine the new electron added will be accommodated in $2 \mathrm{p}$ sub-shell while in chlorine the new electron goes to $3 \mathrm{p}$ subshell. The size of the ${ }^{2 \mathrm{p}}$ subshell is smaller than the size of ${ }^{3 \mathrm{p}}$ sub shell. So, the added electron in $2 \mathrm{p}$ sub shell experiences strong inter-electronic repulsion which is comparatively less in the case of $3 \mathrm{p}$ sub shell of chlorine. The added electron doesn't feel much nuclear attraction in fluorine, that's why fluorine doesn't easily gain new electrons. Therefore, fluorine has less negative electron gain enthalpy than chlorine.
G. When we move down the group in group 13 due to the inert pair effect for Thallium, $+3$ oxidation state is less stable than its $+1$ oxidation state. In $\mathrm{Tl}\left(\mathrm{NO}_{3}\right)_{3}$ the central atom $\mathrm{TL}$ exist in $+3$ oxidation state which is not much stable so it readily gains two electrons to form compounds in which Thallium is in $+1$ oxidation state that is $\mathrm{TINO}_{3}$.
H. As we move down the group in group 14 size of element increases, electronegativity decreases which results in the decrease of M-M born strength hence the catenation decreases.
I. Complete hydrolysis of $\mathrm{BF}_{3}$ does not occur, rather it gets partially hydrolysed. $\mathrm{BF}_{3}$ reacts with water to form boric acid and $\mathrm{HF}$ the $\mathrm{HF}$ further reacts with $\mathrm{H}_{3} \mathrm{BO}_{3}$ and prevents Hydrolysation of $\mathrm{BF}_{3}$.
J. In group 14, as we move down the size of elements increases and electronegativity decreases. Silicon does not have ${ }^{\mathrm{sp}}{ }^{2}$ state and does not form ${n-p} \pi$ double bond like carbon does in graphite. Rather silicon exists in ${ }^{s p^{3}}$ hybridisation state which forms 3-D structure more like diamond.
34. Identify the compound A, X and Z in the following reactions:
(i) $A+2 \mathrm{HCl}+5 \mathrm{H}_{2} \mathrm{O} \longrightarrow 2 \mathrm{NaCl}+X$
Ans: $\underset{(\text { Borax })}{\mathrm{Na}_{2} \mathrm{~B}_{4} \mathrm{O}_{7}}+2 \mathrm{HCl}+5 \mathrm{H}_{2} \mathrm{O} \rightarrow 2 \mathrm{NaCl}+\underset{\text { (Boric acid) }}{\left(\mathrm{H}_{3} \mathrm{BO}_{3}\right.}$
(ii) $X \stackrel{\Delta}{370 \mathrm{~K}} \rightarrow \mathrm{HBO}_{2} \underset{>370 \mathrm{~K}}{\stackrel{\Delta}{\longrightarrow}} Z$
Ans: $\underset{(X)}{\mathrm{H}_{3} \mathrm{BO}_{3}} \stackrel{\Delta .370 \mathrm{~K}}{\longrightarrow} \underset{\text { Metaboric acid }}{\mathrm{HBO}_{2}}+\mathrm{H}_{2} \mathrm{O}$
$4 \mathrm{HBO}_{2} \underset{-\mathrm{H}_{2} \mathrm{O}}{\stackrel{\Delta, 370 \mathrm{~K}}{\longrightarrow}} \underset{\text { Tetraboric acid }}{\left[\mathrm{H}_{2} \mathrm{~B}_{4} \mathrm{O}_{7}\right]} \stackrel{\text { Red heat }}{\longrightarrow} \underset{\text { Boric trioxide(Z) }}{2 \mathrm{~B}_{2} \mathrm{O}_{3}}+\mathrm{H}_{2} \mathrm{O}$
35. Complete the following chemical equations:
$Z+3 \mathrm{LiAlH}_{4} \longrightarrow X+3 \mathrm{LiF}+3 \mathrm{AlF}_{3}$
$X+6 \mathrm{H}_{2} \mathrm{O} \longrightarrow Y+6 \mathrm{H}_{2}$
$X+3 \mathrm{O}_{2} \longrightarrow \mathrm{B}_{2} \mathrm{O}_{3}+3 \mathrm{H}_{2} \mathrm{O}$
Ans:
$\underset{(Z)}{4 \mathrm{BF}_{3}+3 \mathrm{LiAlH}_{4}} \longrightarrow \underset{(X)}{2 \mathrm{~B}_{2} \mathrm{H}_{6}+3 \mathrm{LiF}+3 \mathrm{AlF}_{3}}$
$\underset{(X)}{\mathrm{B}_{2} \mathrm{H}_{6}}+6 \mathrm{H}_{2} \mathrm{O} \longrightarrow \underset{(Y)}{2 \mathrm{H}_{3} \mathrm{BO}_{3}}+6 \mathrm{H}_{2}$
$\underset{(X)}{\mathrm{B}_{2} \mathrm{H}_{6}}+3 \mathrm{O}_{2} \stackrel{\Delta}{\longrightarrow} \mathrm{B}_{2} \mathrm{O}_{3}+3 \mathrm{H}_{2} \mathrm{O}$
Match Type
In the following questions more than one correlation is possible between options of Column I and Column II. Make as many correlations as you can.
36. Match the species given in Column I with the properties mentioned in Column II.
Column I | Column II |
(i) $\mathrm{BF}_{4}^{-}$ | (a) Oxidation state of central atom is +4 |
(ii) $\mathrm{AlCl}_{3}$ | (b) Strong oxidising agent |
(iii) SnO | (c) Lewis acid |
(iv) PbO2 | (d) Can be further oxidised |
(e) Tetrahedral shape |
Ans:(i)-(e), (ii)-(c), (iii)-(d), (iv)-(a) & (b)
Explanation:
(i) $\mathrm{BF}_{4}^{-}$, boron having four sigma bonds with four fluorine atoms; this corresponds to sp $^{3}$ hybridisation and tetrahedral shape.
(ii) $\mathrm{AlCl}_{3}$, electron deficient compound having six electrons in its octet hence acts as the Lewis acid.
(iii) $\mathrm{SnO}$ can be further oxidised as in $\mathrm{SnO}, \mathrm{Sn}$ is and $+2$ oxidation state and can extend its oxidation state up to $+4$.
(iv) $\mathrm{PbO}_{2}$, lead is in $+2$ oxidation state and can be reduced to its most stable oxidation state of $+2$. Hence $\mathrm{PbO}_{2}$ acts as a strong oxidising agent.
37. Match the species given in Column I with properties given in Column II.
Column I | Column II |
(i) Diborane | (a) Used as a flux for soldering metals |
(ii) Gallium | (b) Crystalline form of silica |
(iii) Borax | (c) Banana bonds |
(iv) Aluminosilicate | (d) Low melting, high boiling, useful for measuring high temperatures |
(v) Quartz | (e) Used as catalyst in petrochemical industries. |
Ans: $\mathrm{(i)}\rightarrow(\mathrm{e}),(\mathrm{ii}) \rightarrow(\mathrm{c}),(\mathrm{iii}) \rightarrow(\mathrm{d}),(\mathrm{iv}) \rightarrow(\mathrm{a})(\mathrm{~b}))$
Explanation:
(i) $\mathrm{BF}_{4}^{-}$, boron having four sigma bonds with four fluorine atoms this corresponds to $\mathrm{sp}^{3}$ hybridisation and tetrahedral shape.
(ii) $\mathrm{AlCl}_{3}$, electron deficient compound having six electrons in its octet hence acts as the Lewis acid.
(iii) $\mathrm{SnO}_{\text {can be further oxidized as in }} \mathrm{SnO}, \mathrm{Sn}$ is and $+2$ oxidation state and can extend its oxidation state up to $+4$.
(iv) $\mathrm{PbO}_{2}$, lead is in $+2$ oxidation state and can be reduced to its most stable oxidation state of $+2$. Hence $\mathrm{PbO}_{2}$ act as strong oxidizing agent.
38. Match the species given in Column I with the hybridisation given in Column II.
Column I | Column II |
(i) Boron in $\left[\mathrm{B}(\mathrm{OH})_{4}\right]^{-}$ | (a) sp2 |
(ii) Aluminium in $\left[\mathrm{Al}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{3+}$ | (b) sp3 |
(iii) Boron in $\mathrm{B}_{2} \mathrm{H}_{6}$ | (c) sp3d2 |
(iv) Carbon in Buckminsterfullerene | |
(v) Silicon in $\mathrm{SiO}_{4}^{4-}$ | |
(vi) Germanium in $\left[\mathrm{GeCl}_{6}\right]^{2-}$ |
Ans: $(\mathrm{a})-(\mathrm{iv}),(\mathrm{b})-(\mathrm{i}),(\mathrm{iii}) \&(\mathrm{v}),(\mathrm{c})-(\mathrm{ii}),(\mathrm{vi})$
(i) Boron in $\left[\mathrm{B}(\mathrm{OH})_{4}\right]_{1}^{-}$
In $\left[\mathrm{B}(\mathrm{OH})_{4}\right]^{-}$, boron is the central atom, surrounded by only four bond pairs.
(ii) Aluminium in $\left[\mathrm{Al}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{3+}$
$\ln \left[\mathrm{Al}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{3+}$
Al has a coordination number of 6 and a hexagonal geometry of octahedral.
(iii) Boron in $\mathrm{B}_{2} \mathrm{H}_{6}$
Each B atom in $\mathrm{B}_{2} \mathrm{H}_{6}$ bonds using $s p^{3}$ hybrid orbitals. One of the four $s p^{3}$ hybrids on each B atom lacks an electron.
(iv) Carbon in Buckminsterfullerene
All of the carbon atoms are the same and undergo $s p^{2}$ hybridization.
Each carbon atom establishes three sigma bonds with the other three carbon atoms.
(v) Silicon in $\mathrm{SiO}_{4}^{4-}$
The basic structural unit of silicates is a silicon atom bonded to four oxygen atoms in a tetrahedron fashion.
(vi) Germanium in $\left[\mathrm{GeCl}_{6}\right]^{2-}$
In $\left[\mathrm{GeCl}_{6}\right]^{2-}$, Ge has coordination number 6 and it has octahedral geometry and central atom Ge is $s p^{3} d^{2}$ hybridized.
Assertion and Reason Type
In the following questions a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct option out of the choices given below each question.
39. Assertion (A): If aluminium atoms replace a few silicon atoms in three dimensional network of silicon dioxide, the overall structure acquires a negative charge.
Reason (R): Aluminium is trivalent while silicon is tetravalent.
(A) Both A and R are true and R is the correct explanation of A.
(B) Both A and R are true but R is not the correct explanation of A.
(C) Both A and R are not correct.
(D) A is not correct but R is correct.
Ans: (A)
Explanation:
In aluminium silicate, silicon is doped with group 13 elements and as aluminium is trivalent and silicon is tetravalent so, on replacing aluminium by Silicon, a negatively charged structure will be obtained. Both the statements assertion and reason are correct and reason is the correct explanation of assertion.
40. Assertion (A): Silicones are water repelling in nature.
Reason (R): Silicones are organosilicon polymers, which have (-R $_{2}$ SiO-) as repeating unit.
(A) Both A and R are true and R is the correct explanation of A.
(B) Both A and R are true but R is not the correct explanation of A.
(C) Both A and R are not correct.
(D) A is not correct but R is correct.
Ans: (B)
Explanation: Silicones are a class of silicon polymers that contain the repeating unit (-R $_{2}$ SiO-). Silicones are water repellent in nature because they are surrounded by non-polar alkyl groups, so A and R are both correct, but R is not the correct explanation of A.
Long Answer Type
41. Describe the general trends in the following properties of the elements in Groups 13 and 14.
(i) Atomic size
Ans: Grp-13 The atomic size of the boron family follows the irregular trend. Generally, down the group the size increases but Gallium has a smaller atomic radius than Aluminium due to the poor shielding effect of 3d-orbitals.
Order: B<Ga<Al<In<Ti
Grp-14 The size of the carbon family is smaller than the modern family and as we move down the group the atomic size increases regularly. The increase in covalent radius from carbon to silicon is prominent while from Silicon to lead a small increase in covalent radius is observed; this is due to the presence of completely filled D and f-orbital in the heavier members.
Order: C<Si<Ge<Sn<Pb
(ii) Ionization enthalpy
Ionization enthalpy: In group 13, the standardised trend of decrease of Ionisation enthalpy is not monitored.
As we move from Boron to Aluminium the atomic size increases and ionisation enthalpy decreases but when we move ahead from Aluminium to Gallium, the screening effect of 3d electrons comes into play. The poor shielding effect of the electrons lead to the increase in nuclear charge on the valence electrons and results in increase of ionisation enthalpy.
Moving from gallium to Indium, due to The shielding effect of 4d electrons the ionisation enthalpy decreases.
From Indium to thallium, 4f electrons come into action their poor shielding effect increases the effective nuclear charge on valence electrons hence the ionisation of Thallium energy further increases.
The decreasing order of ionization enthalpy for group-13 elements will be: B>Ti>Ga>Al>In
As we move down in group 14 the ionisation enthalpy decreases in the order: C>Si>Ge>Pb>Sn
On moving down the group the size of the atom increases which results in the large decrease in ionisation energy from carbon to silicon but as we move from silicon to heavier metals small decrease in the ionisation energy is observed. This is mainly due to the less screening effect of d-electrons in germanium and tin, and due to fully filled d & f electrons in lead.
(iii) Metallic character
Ans: Metallic character
The metallic character in group 13 follows an unusual trend. Metallic character First increases from Boron to aluminium and then decreases from aluminium to thallium. This decrease is majorly due to the poor shielding effect of d and f electrons present in the heavier metals.
In group-14, as we move down the group, the metallic character increases.
(iv) Oxidation states
Ans: Possible oxidation states Group 13 elements are from +3 to -5. But the stability of +1 oxidation state increases down the group due to inert their effect than the stability of +3 oxidation state.
Similarly, in group 14, The common oxidation states shown by the carbon family are +2 and +4. Due to the inert pair effect, stability of +2 oxidation state increases then the stability of +4.
(v) Nature of halides
Ans: Except thallium, All the elements of the boron family combine with three atoms of halogens to form trihalides. All these halides exist as molecular species with hybridisation of ${ }^{\mathrm{sp}}{ }^{2}$. All the trihalides are Lewis acid and their acidic strength of trihalides of boron is in order: $\mathrm{Bf}_{3}<\mathrm{BCl}_{3}<\mathrm{BBr}_{3}<\mathrm{BI}_{3}$ In group-14, except carbon, all group 14 elements on reaction with halogens form tetra halides where central metal atom shows the $s p^{3}$ hybridisation state and corresponding tetrahedral shape.
There are few exceptions also, $\mathrm{SnF}_{4}$ and $\mathrm{Pf}_{4}$ are ionic in nature, while the rest are covalent compounds.
42. Account for the following observations:
(i) $\mathrm{AlCl}_{3}$ is a Lewis acid
Ans:
$\ln \mathrm{a} \mathrm{AlCl}_{3}$, Aluminium is bonded to 3 chlorine atoms through three covalent bonds and six electrons are shared in the structure. To have complete Octet, $\mathrm{AlCl}_{3}$ lacks two electrons \& hence act as an electron acceptor substance or Lewis acid.
(ii) Though fluorine is more electronegative than chlorine yet $\mathrm{BF}_{3}$ is a weaker Lewis acid than $\mathrm{BCl}_{3}$
Ans: Undoubtedly, fluorine has more electronegativity than chlorine, $\mathrm{BF}_{3}$ is stronger Lewis acid than $\mathrm{BCl}_{3}$ because of $\mathrm{n}-\mathrm{p} \pi$ back bonding in $\mathrm{BF}_{3}$, both the constituting atoms boron and fluorine are involve p-orbital in back bonding. On moving down the group, the size of halogen atoms increases, back bonding decreases and Lewis acid character also decreases.
(iii) $\mathrm{PbO}_{2}$ is a stronger oxidising agent than $\mathrm{SnO}_{2}$.
Ans: $\mathrm{PbO}_{2}$, lead is in $+2$ oxidation state and can be reduced to its most stable oxidation state of +2. Hence $\mathrm{PbO}_{2}$ can be further exidised or act as a strong oxidising agent. and in $\mathrm{SnO}$, $\mathrm{Sn}$ is and $+2$ oxidation state and can extend its oxidation state up to $+4 $. Thus, $\mathrm{SnO}$ can be used as a reducing agent.
(iv) The $+1$ oxidation state of thallium is more stable than its $+3$ state.
Ans: As we move down the group in group 13 the participation of s-electrons in bond formation decreases the primary reason behind this is the inert pair effect. In this the p-electrons take part in bond formation and more energy is required to unpair the valence electrons to make them participate in bonding. Due to this the lower oxidation state of elements becomes stable than the higher oxidation state. As for thallium, $+1$ oxidation state is more stable than $+3$.
43. When aqueous solution of borax is acidified with hydrochloric acid, a white crystalline solid is formed which is soapy to touch. Is this solid acidic or basic in nature? Explain.
Ans: When aqueous solution of borax is acidified with hydrochloric acid, boric acid is produced. As the name says, boric acid is acidic in nature but weak acid. Unlike protonic acid, boric acid is monobasic acid,
It accepts electrons from the hydroxyl group of water and forms $\left[\mathrm{B}(\mathrm{OH})_{4}\right]^{-}$.
44. Three pairs of compounds are given below. Identify that compound in each of the pairs which has group 13 elements in a more stable oxidation state. Give reason for your choice. State the nature of bonding also.
(i) $\mathrm{TlCl}_{3}, \mathrm{TlCl}$
Ans:
$\mathrm{TlCl}_{\text {is more stable than }} \mathrm{TlCl}_{3}$, due to inert pair effect, $+1$ oxile. oxidation state.
(ii) $\mathrm{AlCl}_{3}, \mathrm{AlCl}$
Ans:
$\mathrm{AlCl}_{3}, \mathrm{Al}^{3+}$ is more stable than aluminum ions in $+1$ state.
(iii) $\mathrm{InCl}_{3}, \mathrm{InCl}$
Ans: Due to the inert pair effect, $+1$ oxidation state is more stable than the $+3$ oxidation state. $\mathrm{So}, \mathrm{InCl}$ is more stable than $\mathrm{InCl}_{3}$.
45. $\mathrm{BCl}_{3}$ exists as monomer whereas $\mathrm{AlCl}_{3}$ is gimerised through halogen bridging. Give reason. Explain the structure of the dimer of $\mathrm{AlCl}_{3}$ also.
Ans: Both the compounds, $\mathrm{BCl}_{3}$ and $\mathrm{AlCl}_{3}$ are electron deficient compounds. In $\mathrm{BCl}_{3}$, boron is smaller in size and cannot assemble four big chlorine atoms near it causing steric hindrance and making it unstable.
Hence, $\mathrm{BCl}_{3}$ exists as a monomer only.
In $\mathrm{AlCl}_{3}$, aluminum has $3 \mathrm{p}$-orbitals through which chlorine atoms can be accommodated easily to complete its octet and dimer is formed.
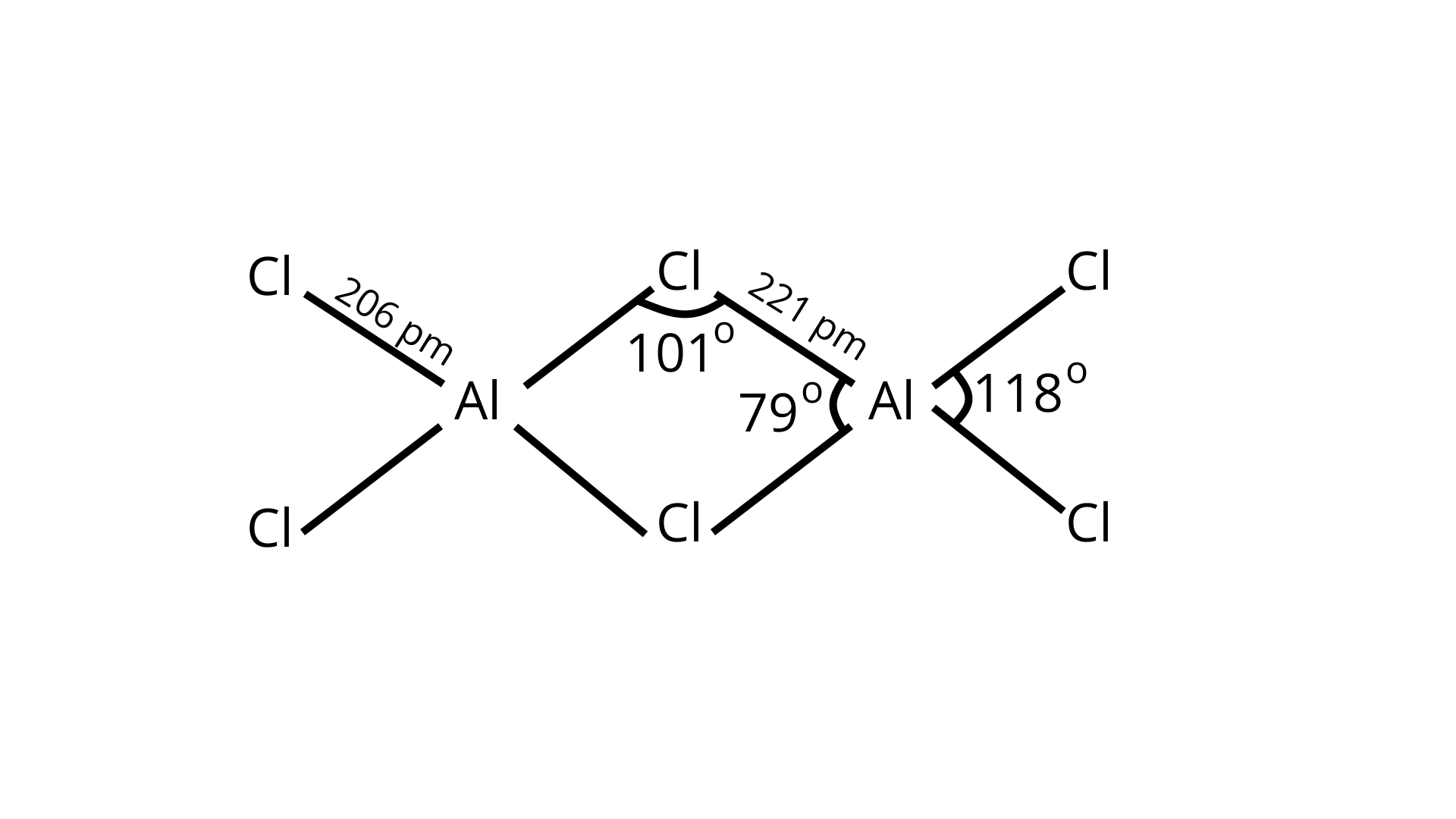
46. Boron fluoride exists as $\mathrm{BF}_{3}$ but boron hydride doesn't exist as $\mathrm{BH}_{3}$. Give reason. In which form does it exist? Explain its structure.
Ans: In $\mathrm{BF}_{3}$, due to $\mathrm{n}-\mathrm{p} \pi$ back bonding between the vacant p-orbital of boron and filled p-orbital of fluorine. This $\Pi-p \pi$ back bonding is absent in case of hydrogen as it is a single electron element.
Two $\mathrm{BH}_{3}$ molecules dimerise to form diborane.
In $\mathrm{B}_{2} \mathrm{H}_{6}$ There are two types of hydrogens present.
(I) Four hydrogens that are terminally bonded to each of two boron atoms.
(II) Two hydrogens that are bonded to both boron atoms forming a bridge in between.
The four terminal hydrogen atoms and two boron atoms lie in the same plane while bridging hydrogen lies in a plane perpendicular to them.
Two hydrogens forming a bridge in $\mathrm{B}_{2} \mathrm{H}_{6}$ are peculiar in bonding and can be termed as 3 -centered-2-electron bond or banana bond. $1 s_{\text {orbital }}$ of each hydrogen overlaps with the hybrid orbital of one of the boron then delocalising the $2 \mathrm{e}^{-}$over three atoms making 3 -centered- 2 -electron bond.
47. (j) What are silicones? State the uses of silicones.
(ii) What are boranes? Give a chemical equation for the preparation of diberane
Ans: (j) Organosilicon polymers having $\left(\mathrm{R}_{2} \mathrm{SiO}_{2}\right)$ as monomer units are called silicones. Silicones contain organic side groups which surround it giving alkane like nature and makes it hydrophobic. Silicones are applicative in electrical insulators, water proofing, sealant. They have been utilized in the biological field in cosmetic implants and other surgeries.
(ii) Boranes correspond to alkane-like compounds of boron. They consist of boron and hydrogen. Most common borane existing is dibecane.
$4 \mathrm{BF}_{3}+3 \mathrm{LiAlH}_{4} \longrightarrow 2 \mathrm{~B}_{2} \mathrm{H}_{6}+3 \mathrm{LiF}+3 \mathrm{AIF}_{3}$
48. A compound (A) of boron reacts with NMe3 to give an adduct (B) which on hydrolysis gives a compound (C) and hydrogen gas. Compound (C) is an acid. Identify the compounds A, B and C. Give the reactions involved.
Ans:
$\mathrm{B}_{2} \mathrm{H}_{6}(\mathrm{~A})+2 \mathrm{NMe}_{3} \rightarrow 2 \mathrm{BH}_{3} \mathrm{NMe}_{3}(\mathrm{~B})$ $\mathrm{BH}_{3} \mathrm{NMe}_{3}+\mathrm{H}_{2} \mathrm{O} \rightarrow \mathrm{H}_{3} \mathrm{BO}_{3}(\mathrm{C})+\mathrm{NMe}_{3}+6 \mathrm{H}_{2}$
(A): $\mathrm{B}_{2} \mathrm{H}_{6}$
(B): ${ }^{2} \mathrm{BH}_{3} \mathrm{NMe}_{3}$
(C): $\mathrm{H}_{3} \mathrm{BO}_{3}$
49. A non-metallic element of group 13, used in making bullet proof vests is an extremely hard solid of black color, It can exist in many allotropic forms and has unusually high melting points. Its trifluoride acts as Lewis acid towards ammonia. The element exhibits a maximum covalency of four. Identify the element and write the reaction of its trifluoride with ammonia. Explain why the trifluoride acts as a Lewis acid.
Ans: Boron is the only non-metallic and extremely hard element in group 13, and it is also used to make bulletproof vests. Boron exists in a variety of allotropic forms. It usually has a high melting point and no d orbital. Using $2 s$ and $2 p$ orbitals, it can achieve a maximum covalency of 4 . Because the octet of boron is not completed in trivalent halides of boron, it acts as Lewis acid. It forms an adduct when it reacts with Lewis base.
$\mathrm{BF}_{3}+\mathrm{NH}_{3} \rightarrow \mathrm{H}_{3} \mathrm{~N}-\mathrm{B}-\mathrm{F}_{3}$
50. A tetravalent element forms monoxide and dioxide with oxygen. When air is passed over a heated element (1273 K), producer gas is obtained. Monoxide of the element is a powerful reducing agent and reduces ferric oxide to iron. Identify the element and write formulas of its monoxide and dioxide. Write chemical equations for the formation of producer gas and reduction of ferric oxide with the monoxide
Ans:
$2 \mathrm{C}(\mathrm{s})+\mathrm{O}_{2}+4 \mathrm{~N}_{2}(\mathrm{~g}) \stackrel{\text { I273k }}{\longrightarrow} 2 \mathrm{CO}(\mathrm{g})+4 \mathrm{~N}_{2}(\mathrm{~g})$
$\mathrm{Fe}_{2} \mathrm{O}_{3}(\mathrm{~S})+3 \mathrm{CO}(\mathrm{g}) \stackrel{\text { heat }}{\longrightarrow} 2 \mathrm{Fe}(\mathrm{S})+3 \mathrm{CO}_{2}$
$\mathrm{C}=\text { tetravalent carbon }$
$\mathrm{CO}=\text { carbon monoxide }$
$\mathrm{Fe}_{2} \mathrm{O}_{3}=\text { ferric oxide }$
$\mathrm{CO}_{2}=\text { carbon dioxide }$
Tetravalent elements i.e. carbon combines with oxygen to produce carbon monoxide. The reaction occurs at high temperatures and in the presence of nitrogen gas, which acts as a producer gas only. It is not consumed in the reaction. The carbon monoxide formed acts as a reducing agent for ferric oxide, reduces the oxidation state of iron from $+3$ to zero, and oxidizes itself to form carbon dioxide.
Summary of Chapter 11 p-Block Elements of Chemistry of Class 12
The Periodic Table is quite huge to learn about the various Elements, therefore for the sake of convenience, it has been divided into four Blocks. These four Blocks are s, p, d and f-Blocks and are divided upon the filling of the particular shell (with Electrons) similar to their names.
The Chapter deals with the p Block Elements of this Periodic Table . The Elements in which the last Electron enters the p-subshell of the outermost energy level are called the p-Block Elements. The Elements from groups 13 to 18 are included in the p Block Elements. These groups are called the Boron family, Carbon family, Nitrogen family, Oxygen family (Chalcogen), Fluorine family (halogens) and Helium family (Noble gases), named after the first Element of the group. Combined there are 31 Elements in total in the p-Block Elements. These 6 groups have one to six Elements in their p orbitals and the s-orbital in them is already filled. The general Electronic configuration of p-Block Elements is ns2 np1-6, where n can vary from 2 to 7. The inner core of the Electronic configuration of Elements may, however, differ.
Chapter 11 of Class 11 Chemistry only discusses the first two families (out of the six groups) in detail (Boron and Carbon family). Students will be able to understand the various concepts from this Chapter from the NCERT Exemplar solution of Class 11 Chemistry Chapter 11 p Block Elements.
FAQs on NCERT Exemplar for Class 11 Chemistry Chapter-11 (Book Solutions)
1. What is the boron and carbon family? Name Elements included in them.
Boron Family: The Boron family is a name given to the Elements of group 13 of the Periodic Table . The Elements included in the Boron Family are Boron (B), Aluminium (Al), Gallium (Ga), Indium (In) and Thallium (Tl). the general Electronic configuration for this group is ns2 np1.
Carbon Family: The Elements of group 14 of the Periodic Table are called the Carbon family, as their first Element is carbon. The Group includes the Elements of Carbon (C), Silicon (Si), Germanium (Ge), Tin (Sn) and Lead (Pb).
2. How many questions are there in NCERT Exemplar for Class 11 Chemistry Chapter 10?
NCERT Exemplar for Class 11 Chemistry for Chapter 11 p-Block Elements includes a total of 50 problems that need to be solved. These questions are related to the various concepts of the Chapter 11 p-Block. The questions focus on various chemical and physical properties of two groups of p Block Elements, boron and carbon family. Students can download solutions to all these NCERT Exemplar in Chapter 11 (p Block) questions from the official website of vedantu. These questions are solved by expert Chemistry teachers from vedantu to help you to comprehend all the important concepts.
3. List the major topics and sub-topics from the Chapter 11 p-Block Elements.
Some of the major concepts in the Chapter 11 p Block Elements of Class 11 are:
Group 13 Elements: The Boron Family
Electronic Configuration of group 13
Electronegativity
Physical Properties
Chemical Properties
Anomalous behaviour Of Boron
Important Compounds Of Boron
Uses Of Boron And Aluminium And Their Compounds
Group 14 Elements: The Carbon Family
Electronic Configuration of group 14 Elements
Electronegativity
Physical Properties
Chemical Properties
Anomalous Behaviour Of Carbon
Allotropes Of Carbon
Uses Of Carbon
Some Important Compounds Of Carbon And Silicon.
4. Explain the Allotropes of carbon as mentioned in Chapter 11 p Block Elements.
Allotropes of an Element mean the different forms of the same Elements, which have distinct physical properties but nearly the same chemical properties.
Carbon has a lot of allotropes, but they can be divided into two types.
Crystalline: These forms of carbon have a well-defined structure. Examples of these types of allotropes will be Diamond and Graphite.
Amorphous: Much of the carbon’s allotropes are of Amorphous forms such as Coal, Charcoal, Coke and gas carbon, etc.
5. Write down some uses of Boron and Aluminium.
Uses of Boron:
Boron is used as a semiconductor in Electronic devices.
It is also used in the steel industry to increase the hardness of steel.
Compounds of boron (such as Borax and Boric acids) are used in the glass manufacturing industry to make heat resistant glass.
Important uses of Aluminium:
As it is light, soft, non-toxic and corrosion-resistant, it is widely used in household utensils and structural metal for various kinds of vehicles.
Used for making Electric power cables
Aluminium foils are being used for making packages for various food materials.

























