Science Notes for Chapter 5 Life Processes Class 10 - FREE PDF Download
CBSE Notes Class 10 Science Chapter 5 - Life Processes - 2025-26
FAQs on CBSE Notes Class 10 Science Chapter 5 - Life Processes - 2025-26
1. What are the four main life processes to focus on for a quick revision of this chapter?
For a quick revision of Class 10 Science Chapter 5, focus on the four essential life processes: Nutrition (how organisms get food), Respiration (how energy is released from food), Transportation (how substances move within the body), and Excretion (how metabolic waste is removed).
2. How can I quickly summarise the key stages of human nutrition for revision?
To revise human nutrition, remember these five key stages: Ingestion (taking in food), Digestion (breaking down complex food in the mouth, stomach, and small intestine), Absorption (absorbing nutrients in the small intestine), Assimilation (using absorbed food for energy and growth), and Egestion (removing undigested waste).
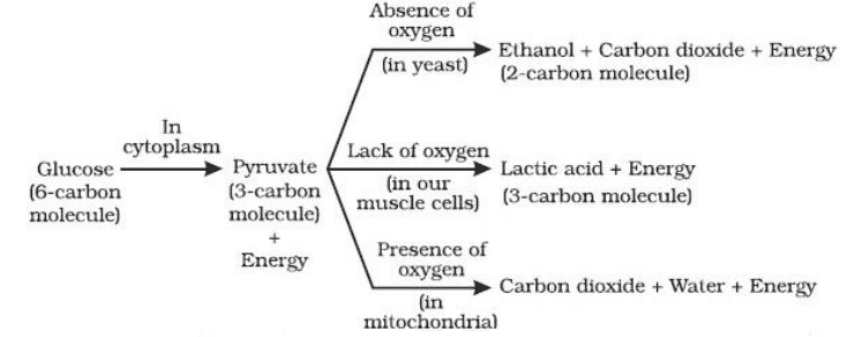
3. What is the main difference between aerobic and anaerobic respiration to remember for exams?
The primary difference to remember is that aerobic respiration occurs in the presence of oxygen, produces a large amount of energy (ATP), and its end products are carbon dioxide and water. In contrast, anaerobic respiration occurs without oxygen, produces less energy, and results in lactic acid (in human muscles) or ethanol and carbon dioxide (in yeast).
4. What is the significance of double circulation in the human heart?
The significance of double circulation is that it ensures the complete separation of oxygenated and deoxygenated blood. This allows for a highly efficient supply of oxygen to the body's cells, which is necessary to support the high energy demands of warm-blooded animals like humans. Blood passes through the heart twice in one complete cycle.
5. How do transport in plants via xylem and phloem differ?
The key difference lies in what they transport and the direction of flow. Xylem transports water and minerals from the roots to all other parts of the plant, which is a unidirectional (upward) flow. Phloem transports food (sucrose) from the leaves to other parts of the plant, like roots and fruits, which is a bidirectional (up and down) flow depending on the plant's needs.
6. What is the functional unit of the kidney, and what are its main functions for revision?
The functional unit of the kidney is the nephron. For revision, remember its two main functions:
- Filtration: It filters blood to remove waste products like urea, forming an initial filtrate.
- Selective Reabsorption: It reabsorbs useful substances like glucose, amino acids, salts, and water back into the blood, concentrating the waste into urine.
7. Why do plants excrete waste products differently than animals?
Plants excrete waste differently because their metabolic processes are much slower and they can reuse some waste products. They lack a specialised excretory system like animals. Key methods include releasing oxygen (a waste product of photosynthesis) via stomata, shedding leaves, storing waste in cell vacuoles, and secreting wastes as resins and gums.
8. What are the common misconceptions to avoid when revising autotrophic and heterotrophic nutrition?
A common misconception is thinking all unicellular organisms are simple eaters. While amoeba uses phagocytosis, the core difference to remember is the source of food. Autotrophs (like plants) produce their own food from inorganic sources (CO₂ and water) using light energy. Heterotrophs (like animals and fungi) obtain energy by consuming organic matter from other organisms. Don't confuse egestion (removing undigested food) with excretion (removing metabolic waste).
9. How can I create a quick concept map to link all four life processes for effective revision?
To create a concept map, start with 'Living Organism' at the centre. Branch out to the four processes:
- Nutrition: Connect this to 'Energy Input' (food).
- Respiration: Link 'Nutrition' to 'Respiration', showing that food is broken down to release 'Energy (ATP)'.
- Transportation: Show how this system delivers nutrients and oxygen from the first two processes to all cells and collects waste.
- Excretion: Connect this to 'Transportation', showing that it removes metabolic waste collected by the transport system.




















 Watch Video
Watch Video