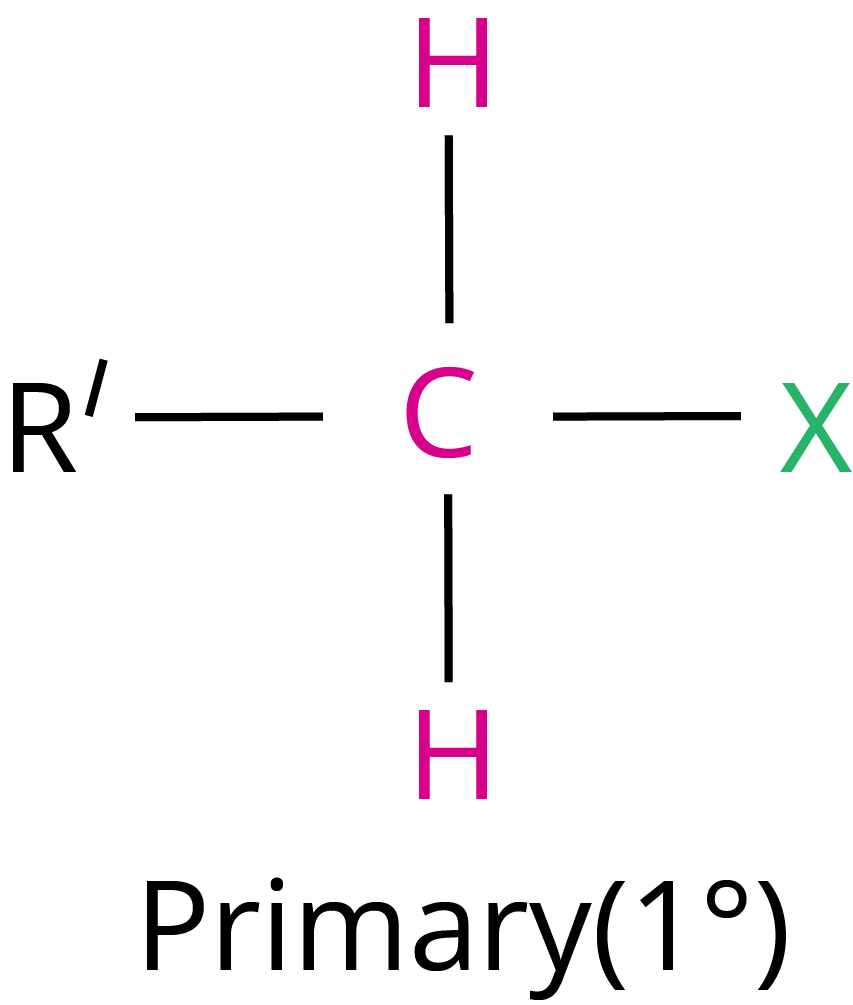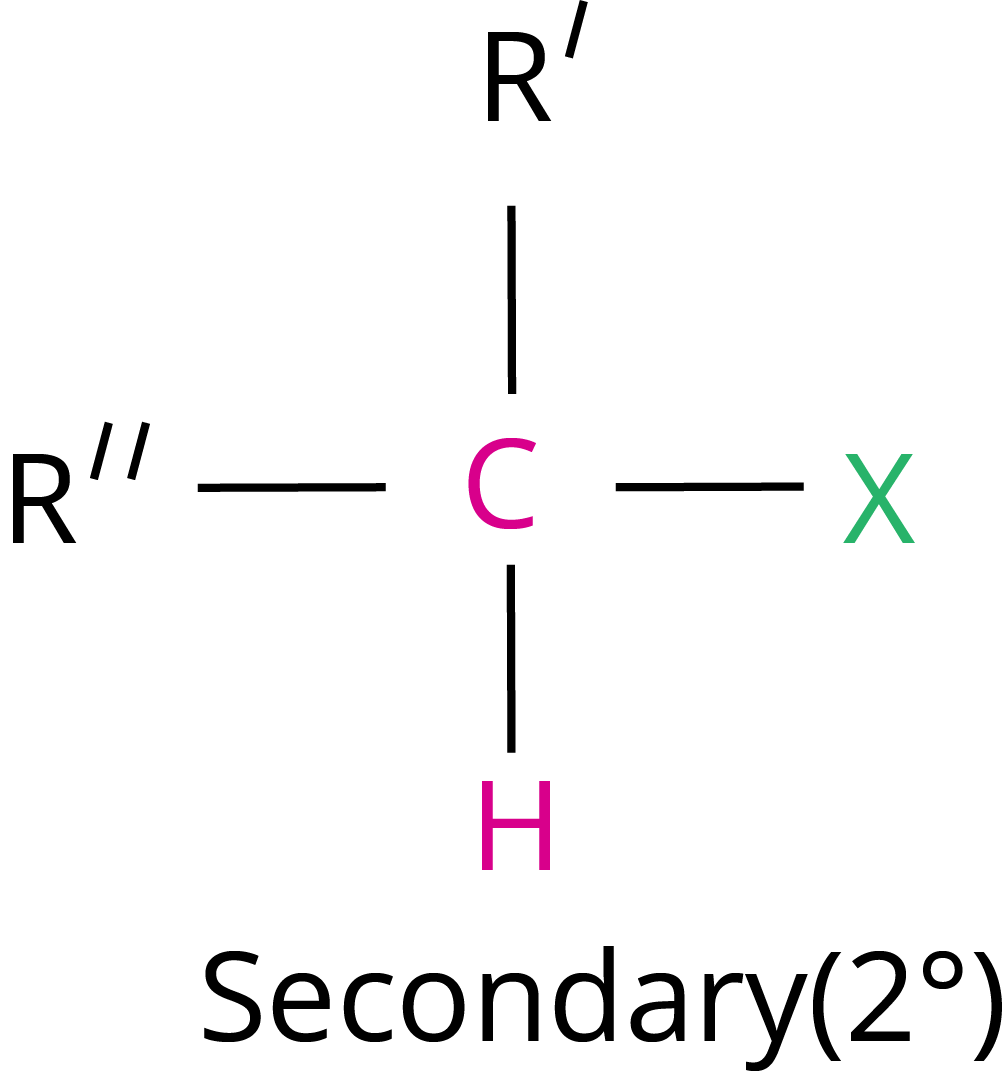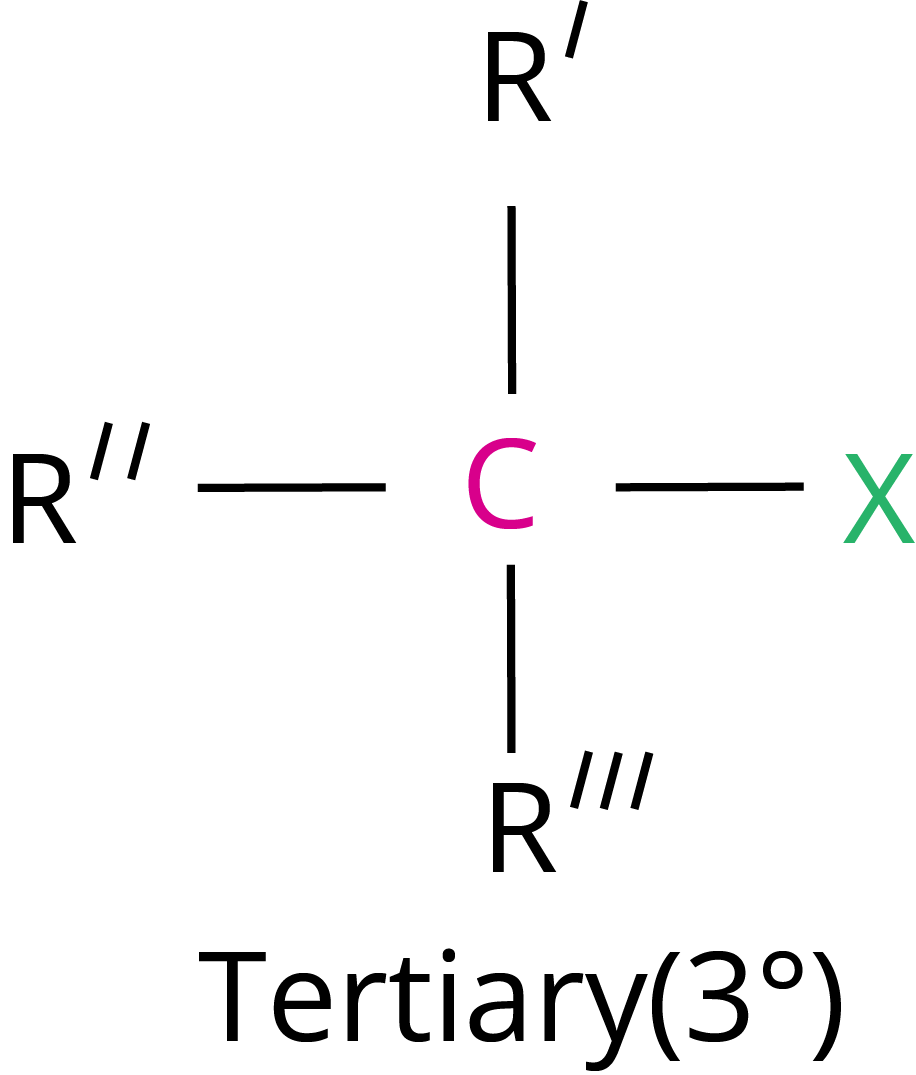Chemistry Haloalkanes and Haloarenes Notes for NEET
Many halogen-containing organic compounds can be found in nature, and some of them can be used in clinical settings. These types of chemicals have a wide range of applications in both business and everyday life. They are utilized as starting materials for the synthesis of a wide range of organic molecules and as solvents for comparatively non-polar chemicals.
In this chemistry haloalkanes and haloarenes notes, we have covered all the important topics like the physical properties of haloalkanes and haloarenes, the chemical properties of haloalkanes and haloarenes and important reactions.
Important Topics of Chemistry Haloalkanes and Haloarenes Notes
Alkyl halide
Allyl halide
Benzyl halide
Vinyl halide
Halo arenes
Swarts reaction
Finkelstein reaction
SN1 reaction
SN2 reaction
Important Definition of Haloalkanes and Haloarenes NCERT Notes
What are Haloalkanes and Haloarenes?
Hydrocarbons whose one or more hydrogen atoms are replaced by an equal number of halogen atoms (F, Cl, Br, or I) are called haloalkanes or haloarenes. If the halogen replaces hydrogen from the aliphatic hydrocarbon then this compound is known as haloalkane while if the halogen replaces hydrogen from aromatic hydrocarbon then it is known as haloarenes.
Nomenclature of Haloalkanes and Haloarenes
In the IUPAC system of nomenclature, R-Cl (alkyl halides) are named as halo substituted hydrocarbons for eg: the IUPAC name for the given compound is: CH3-CH2-Cl (Chloroethane). The common and IUPAC names for mono halogen-substituted benzene derivatives are the same. In the common system, the prefixes o-, m-, and p- are used for halogen derivatives, whereas in the IUPAC system, the prefixes o-, m-, and p- are used. Alkylidene or alkylene dihalides are dihaloalkanes with the same type of halogen atoms.
Physical Properties of Haloalkanes and Haloarenes
When pure, alkyl halides are colourless. When bromides and iodides are exposed to light, they take on a colour.
Many halogen compounds that are volatile have a sweet odour.
At room temperature, methyl chloride, methyl bromide, ethyl chloride, and certain chlorofluoromethanes are gasses. Liquids or solids are the higher members.
Chlorides, bromides, and iodides have far higher boiling points than hydrocarbons with equivalent molecular masses.
Hydrocarbon derivatives like Bromo, iodo, and polychloro are heavier than water.
In water, the haloalkanes are only very weakly soluble. Energy is necessary to overcome the attraction between haloalkane molecules and break the hydrogen bonds between water molecules in order to dissolve haloalkane in water.
Chemical Properties of Haloalkane and Haloarenes
1. Substitution Reaction
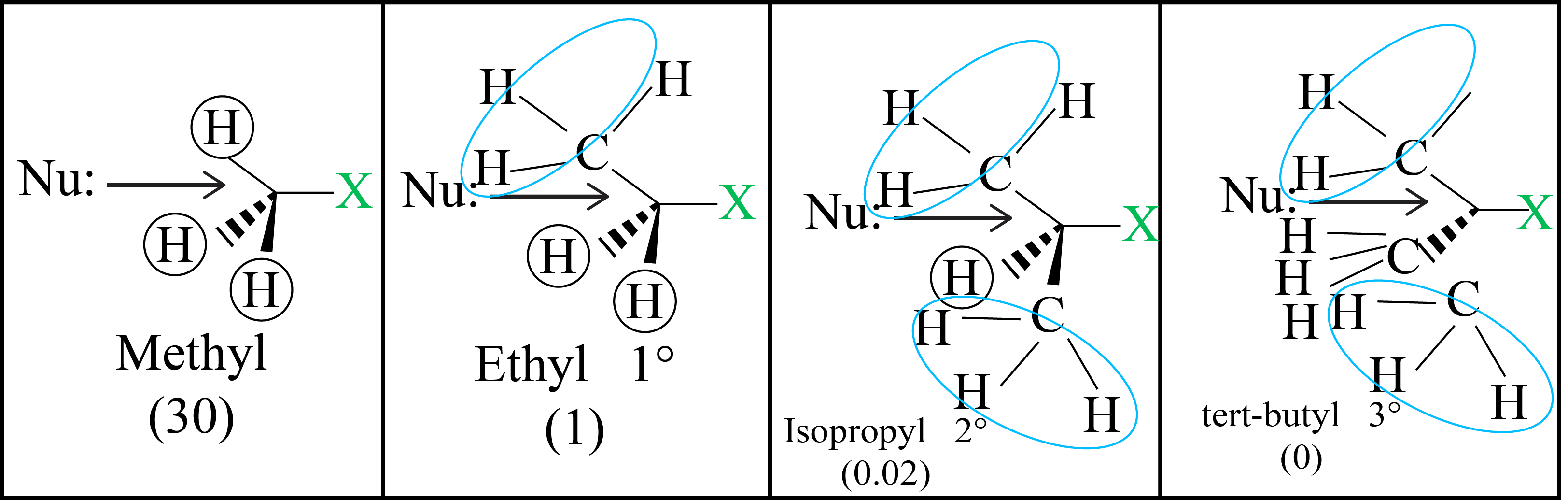
(a) Substitution Nucleophilic bimolecular (SN2) Reaction
The reaction between CH3Cl and hydroxide ions to produce methanol and chloride ions follows second order kinetics, which means that the rate is determined by the concentrations of both reactants.
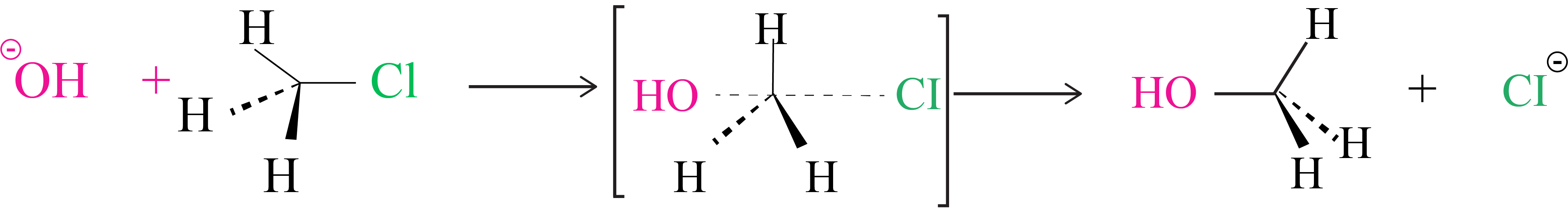
There is no intermediary generated because these two processes occur simultaneously in a single phase. As a result, the attacked group's carbon atom structure inverts.
Because the carbon atom in the transition state is connected to five atoms at the same time, it is unstable.
Because this reaction necessitates the nucleophile to approach the carbon-containing leaving group, bulky substituents on or near the carbon atom have a significant inhibitory effect. As a result, the following is the order of reactivity:
Primary halide > Secondary halide > Tertiary halide.
(b) Substitution reaction SN1
In most cases, SN1 reactions are carried out in polar protic solvents (like water, alcohol, acetic acid, etc.). This reaction follows first-order kinetics, which means that the rate of reaction is determined by the concentration of only one reactant, tert-butyl bromide. It happens in two stages. The polarized C—Br bond is slowly cleaved in step I, yielding a carbocation and a bromide ion. In step II, the carbocation is attacked by a nucleophile to complete the substitution reaction.
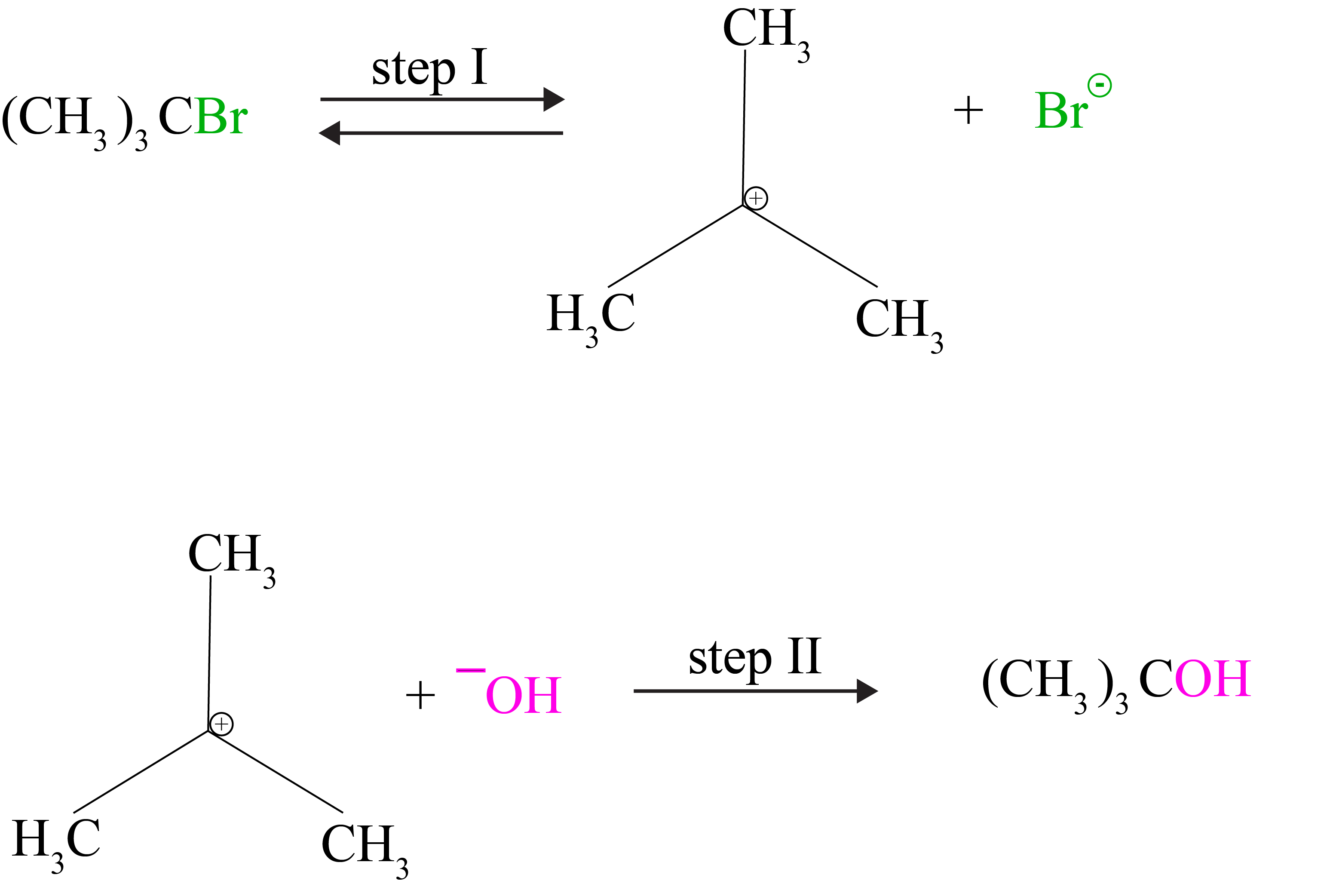
The first step is the slowest and most reversible. Because the reaction rate is determined by the slowest step, the rate is determined solely by the concentration of alkyl halide and not by the concentration of hydroxide ion.
Furthermore, the higher the stability of a carbocation, the easier it will be to generate it from an alkyl halide and the faster the process will be. The sequence of reactivity of alkyl halides towards SN1 and SN2 reactions can be summarized as follows:
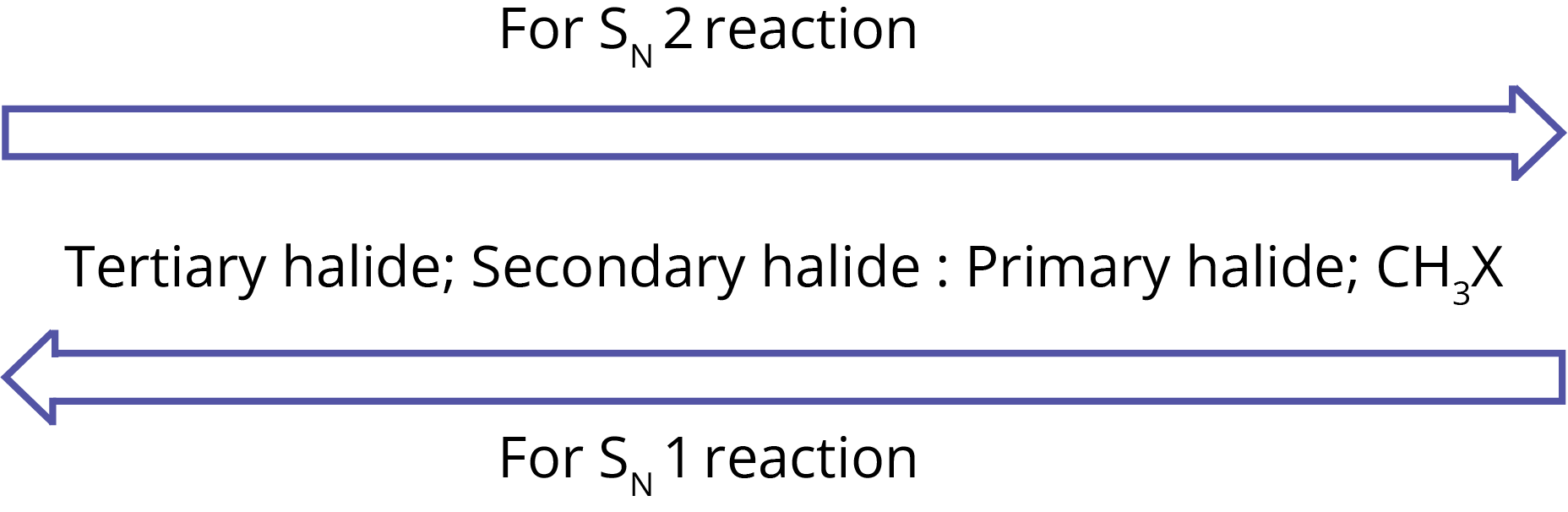
2. Finkelstein Reaction
R-X + NaI → R-I + NaX
x= Cl, Br
3. Swarts Reaction
CH3-Br + AgF → CH3-F + AgBr
3. Elimination Reaction
When a haloalkane containing a -hydrogen atom is heated with an alcoholic potassium hydroxide solution, the hydrogen and halogen atom from the carbon atom is removed. As a result, an alkene is generated as a result of the reaction. Beta hydrogen is eliminated in this reaction, which is known as a beta-elimination reaction in chemistry.
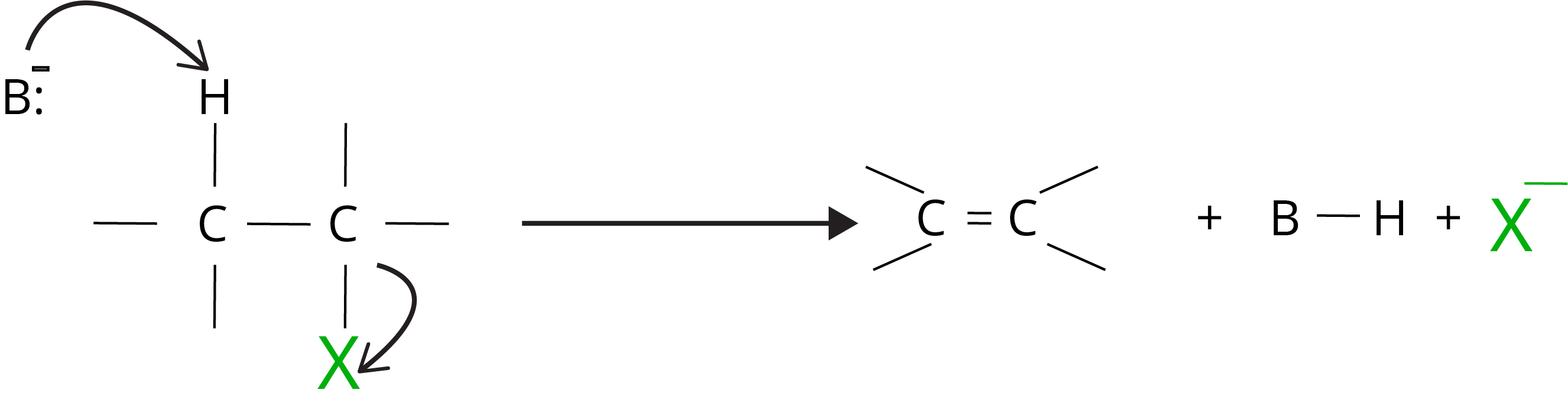
4. Reaction with Metals
The majority of organic chlorides, bromides, and iodides create carbon-metal bonds when they react with particular metals.

5. Wurtz Reaction
In dry ether, alkyl halides react with sodium to produce hydrocarbons with double the number of carbon atoms as the halide. The Wurtz reaction is the name for this reaction.
2RX + 2Na → RR + 2NaX
6. Substitution Reaction in Haloarenes
By heating chlorobenzene in an aqueous sodium hydroxide solution at 623K and 300 atmospheres, chlorobenzene can be transformed to phenol. The reactivity of haloarenes is increased by the presence of an electron-withdrawing group (-NO2) at ortho- and para-positions.
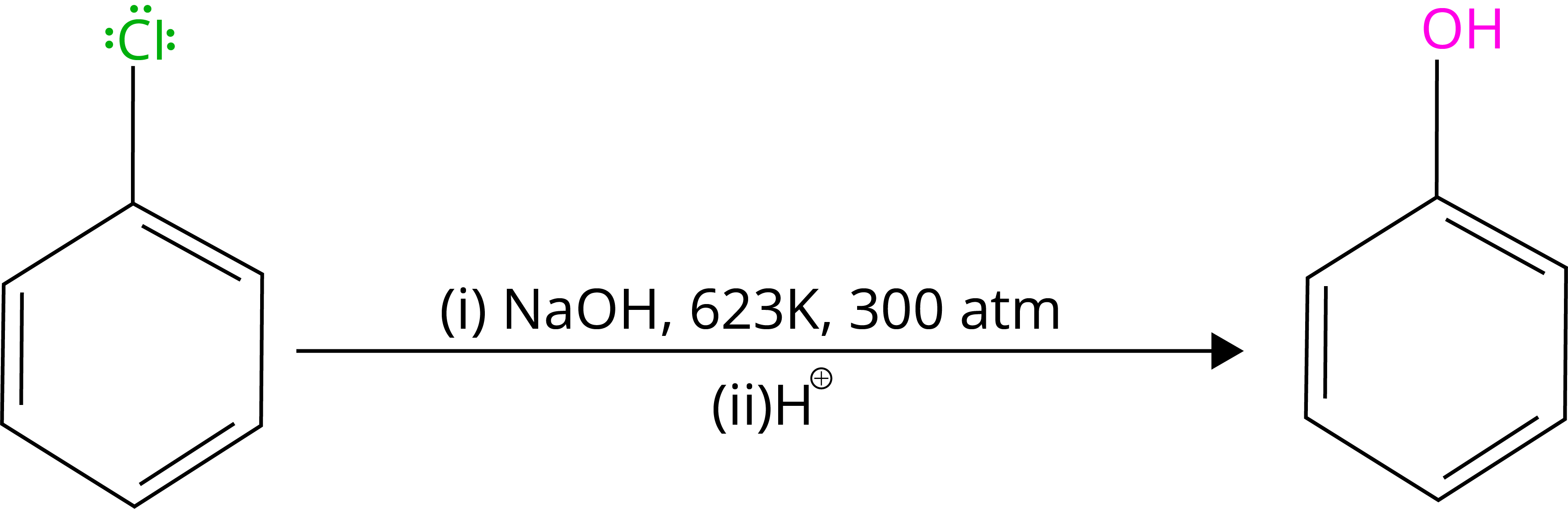
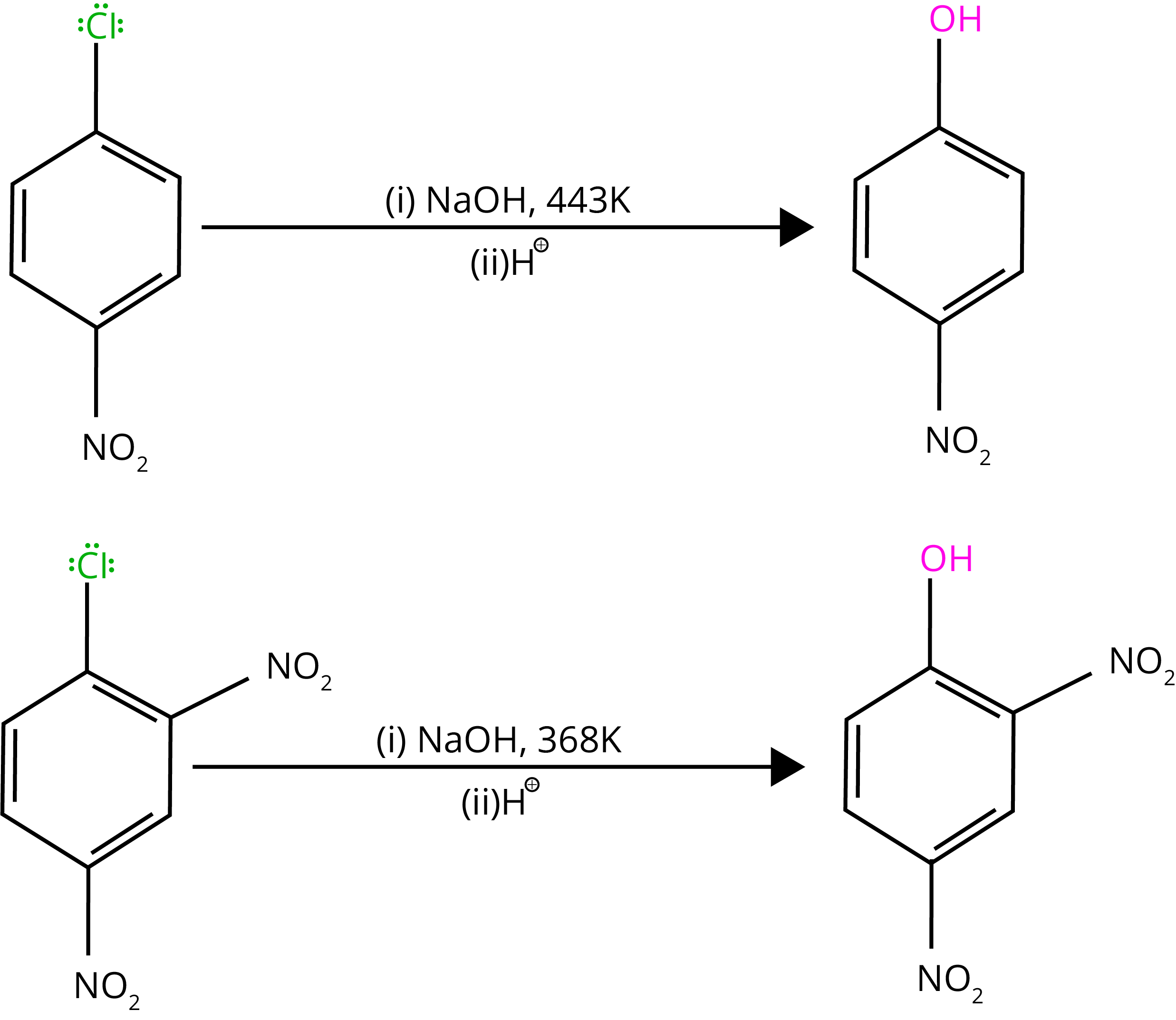
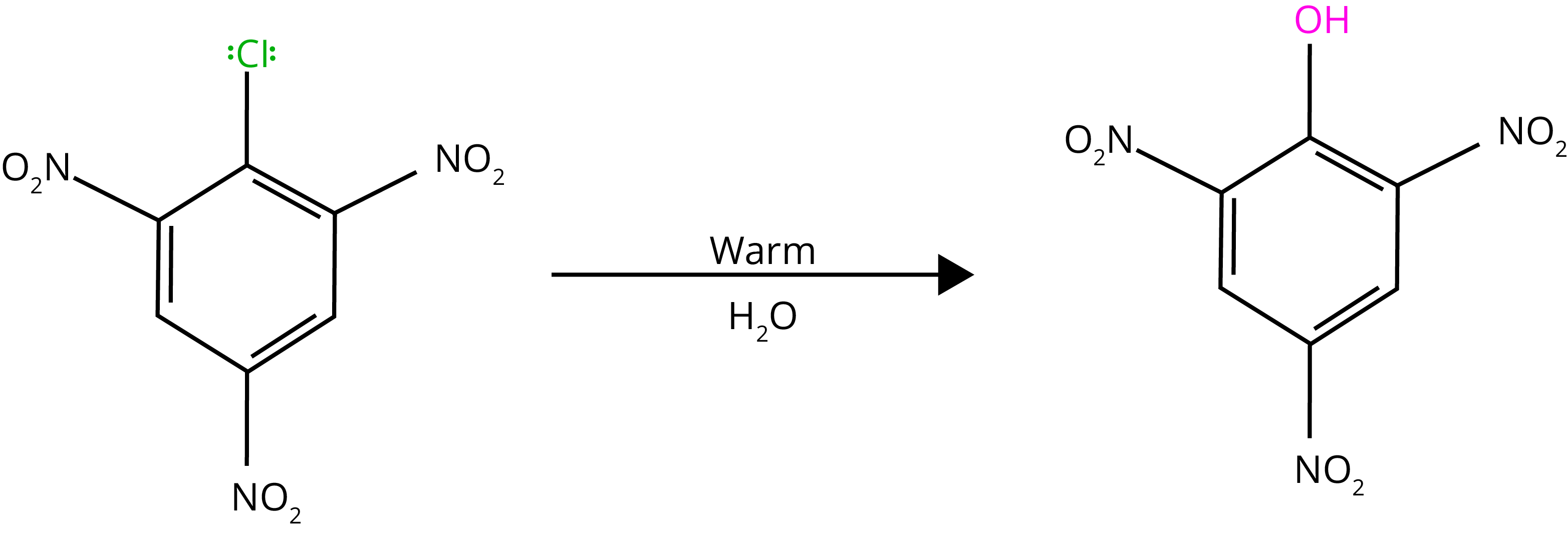
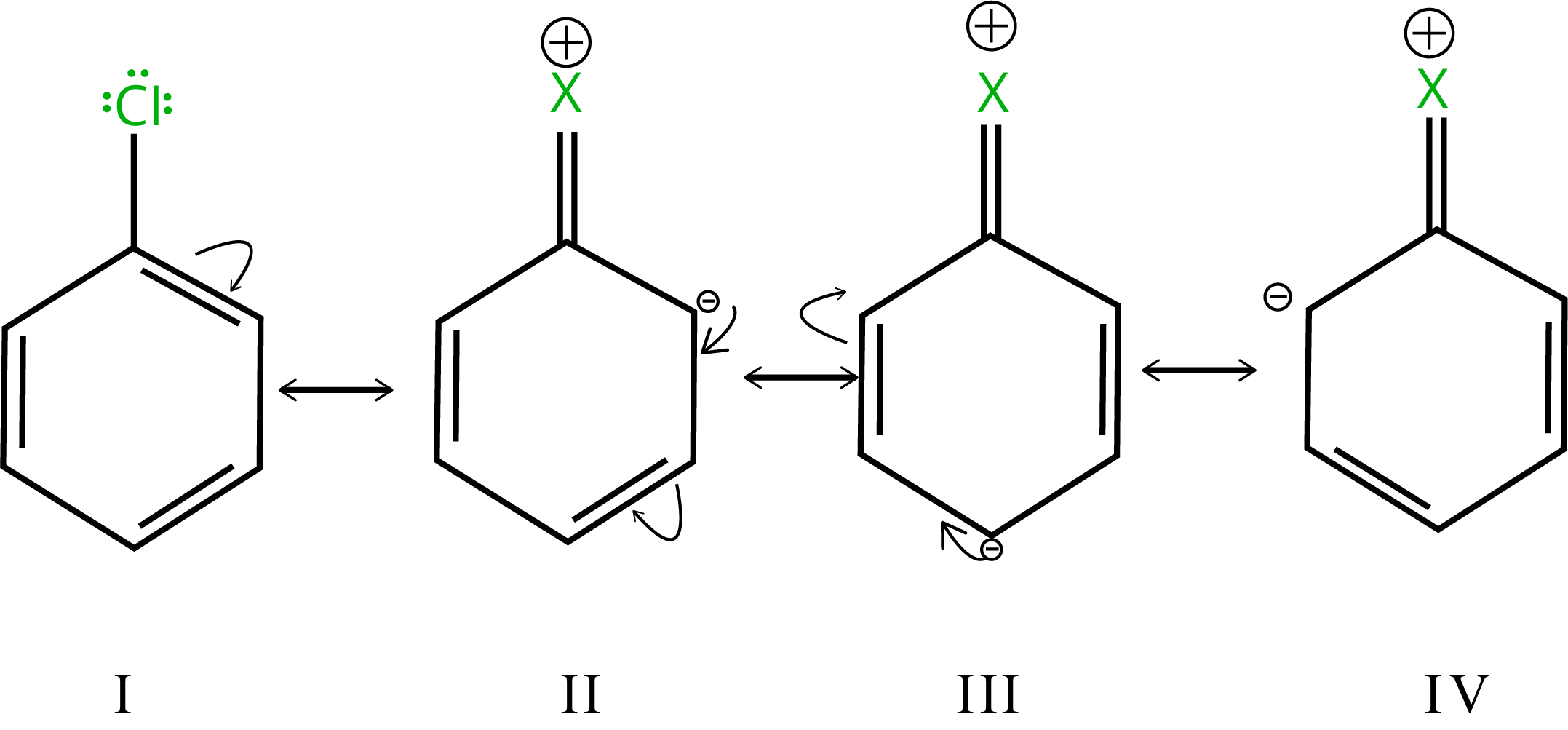
7. Electrophilic Substitution Reactions
The electron density grows higher in ortho- and para-positions than in meta-positions due to resonance.
A. Halogenation Reaction
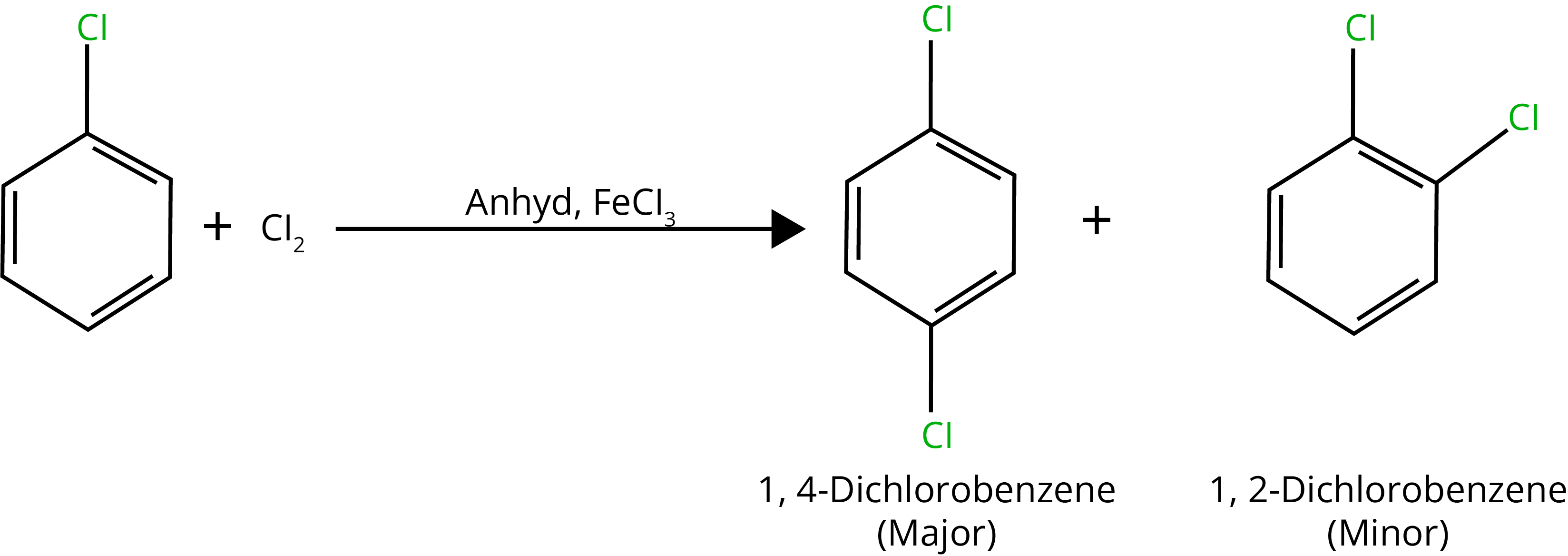
B. Nitration
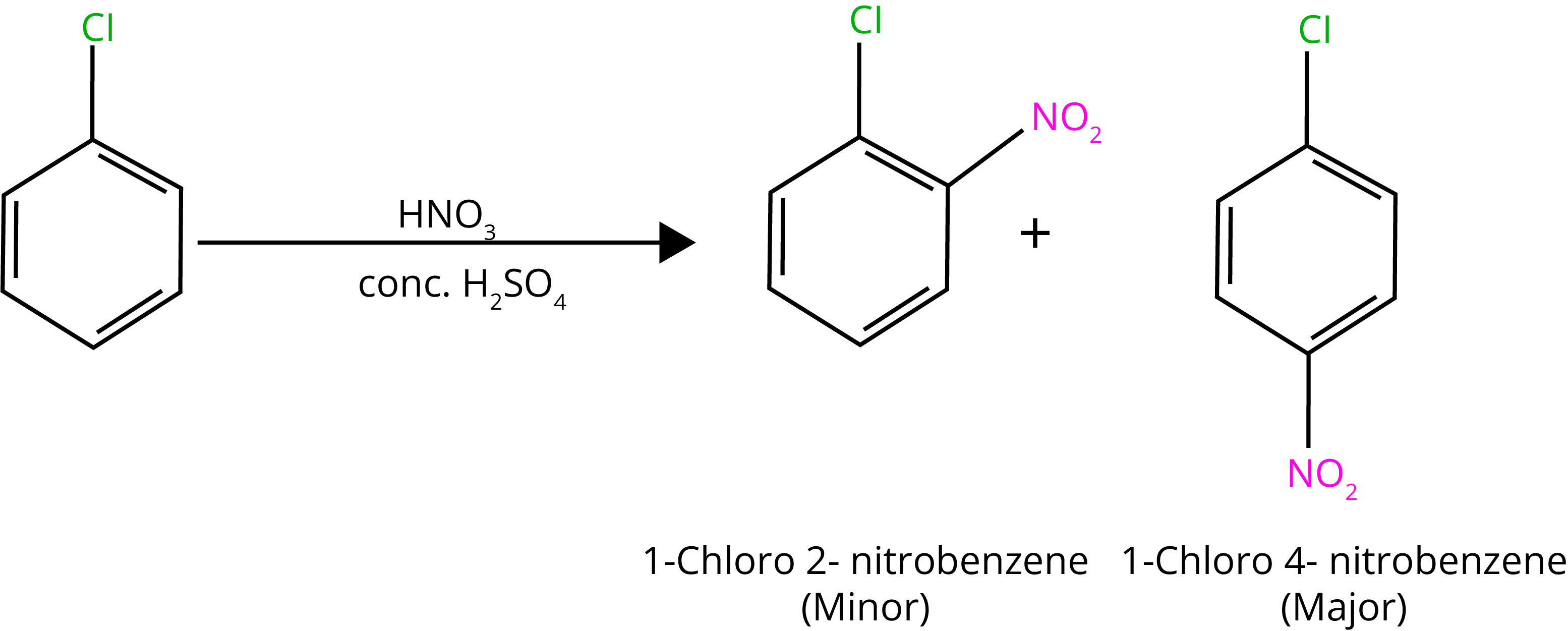
C. Sulphonation
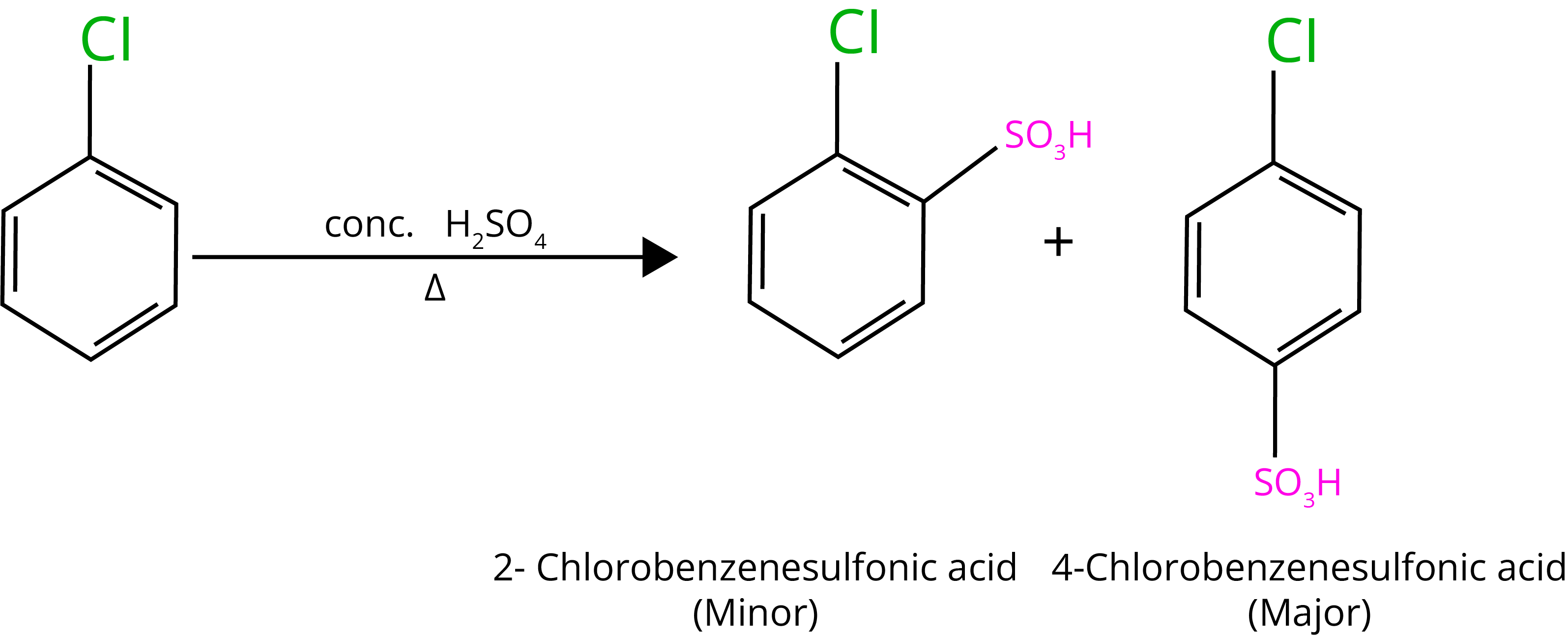
D. Friedel Craft Alkylation
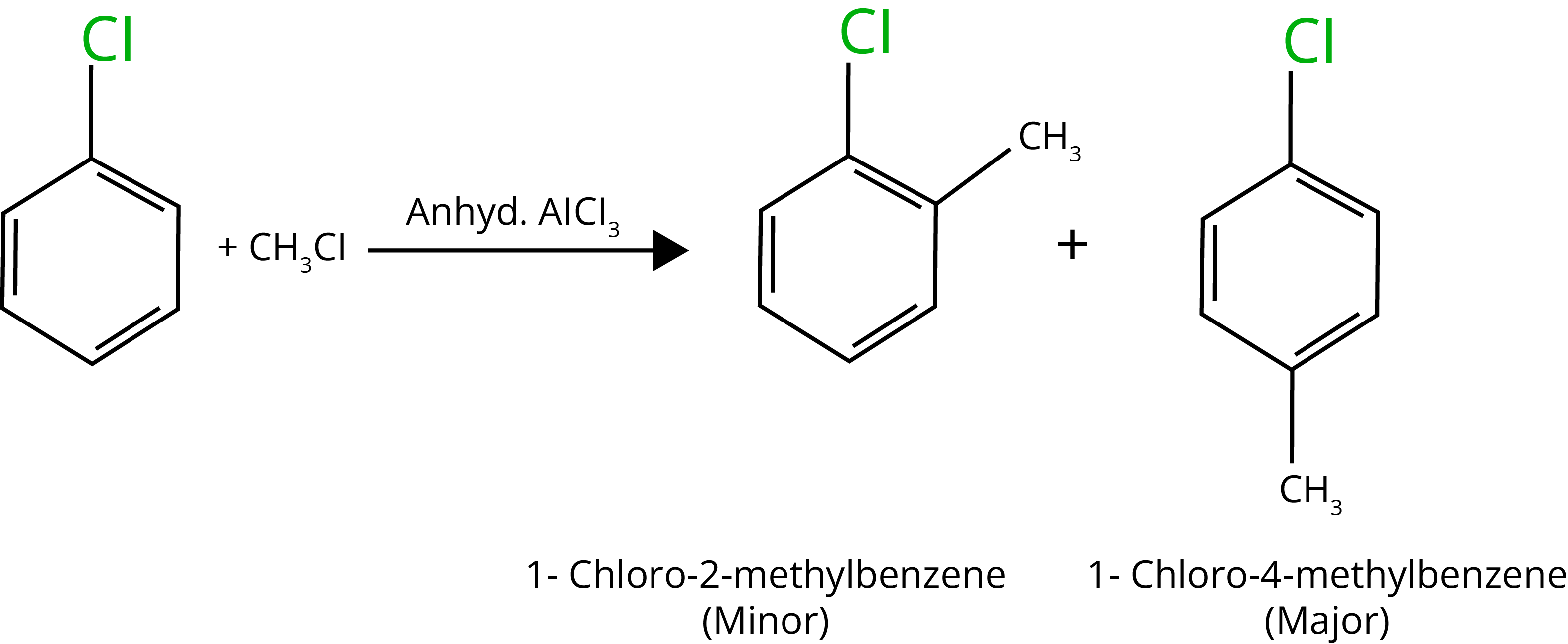
E. Fridel Craft Acylation
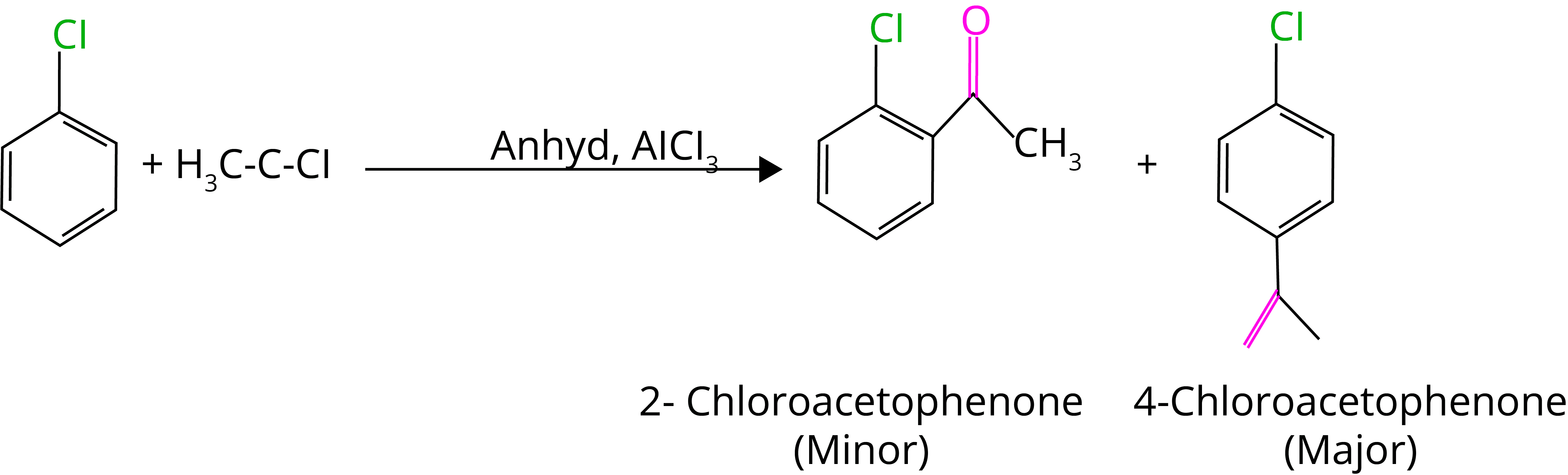
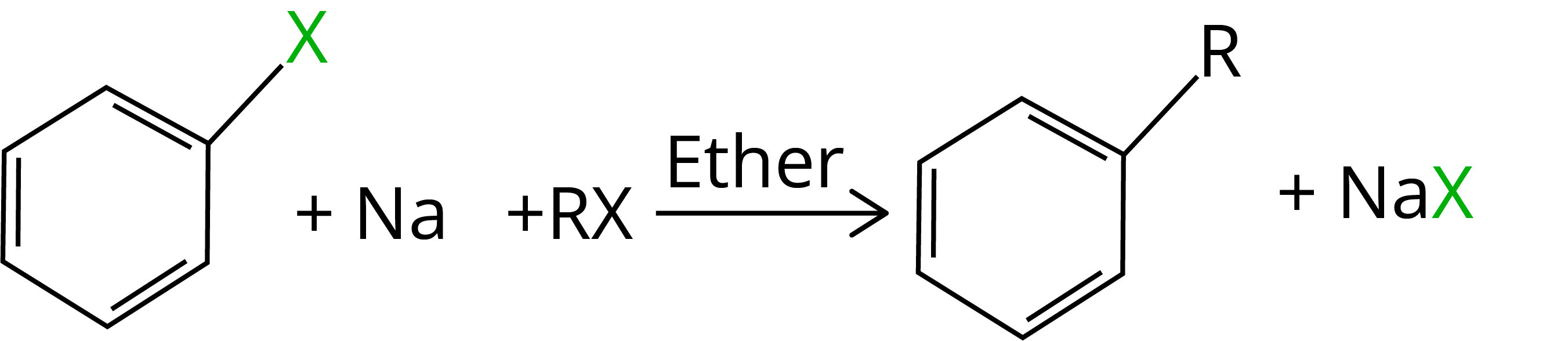
8. Wurtz-fittig Reaction
When an alkyl halide and an aryl halide are combined with sodium in dry ether, the result is an alkyl arene, which is known as the Wurtz-Fittig reaction.
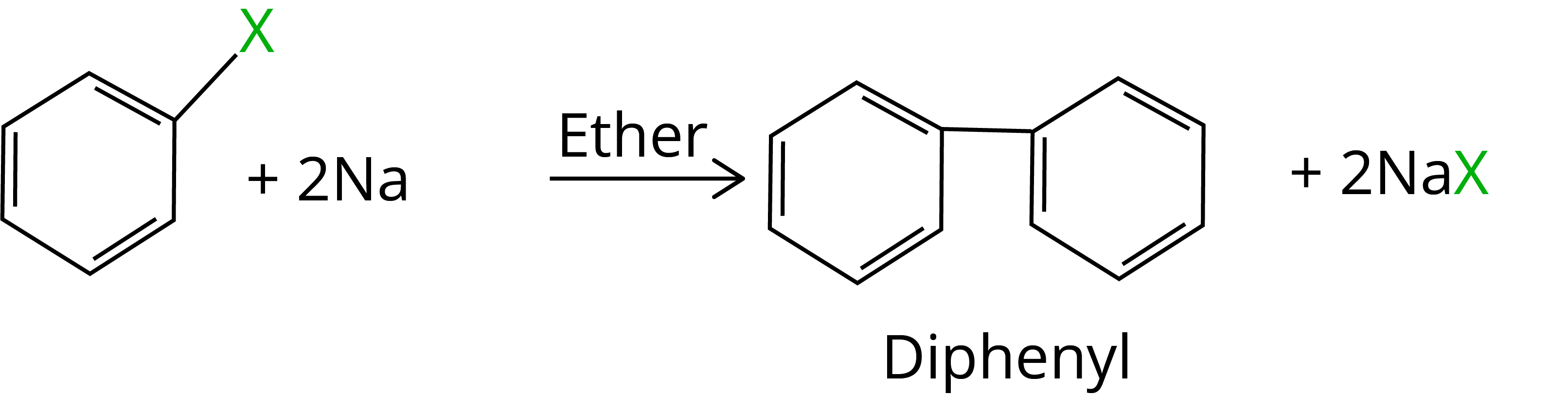
9. Fittig Reaction
When aryl halides are treated with sodium in dry ether, two aryl groups are linked together to form similar compounds. It's known as the Fittig reaction.
Solved Examples/Problems From Chapter
1. Haloalkanes react with KCN to form alkyl cyanides as the main product
while AgCN forms isocyanides as the chief product. Explain.
Ans: KCN is primarily an ionic compound that produces cyanide ions in solution.
Although both carbon and nitrogen atoms can give electron pairs, the attack is focused on the carbon atom rather than the nitrogen atom because the C—C bond is more stable than the C—N bond. However, in nature, AgCN is mostly covalent, and nitrogen is free to contribute an electron pair, resulting in isocyanide as the major product.
Key Points to Remember: KCN is an ionic compound while AgCN is covalent in nature.
2. Identify A and B in the given Reaction:
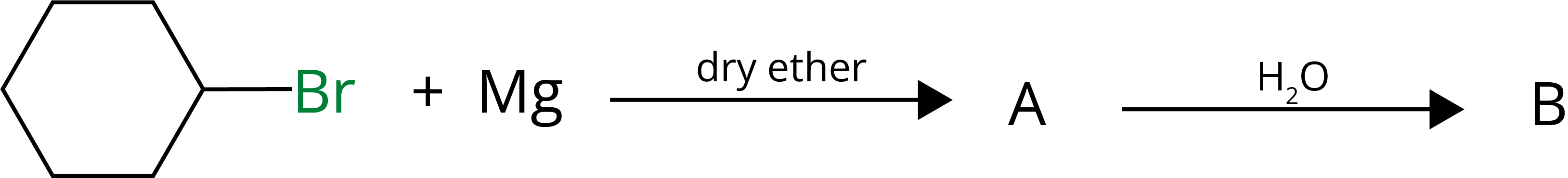
Ans:
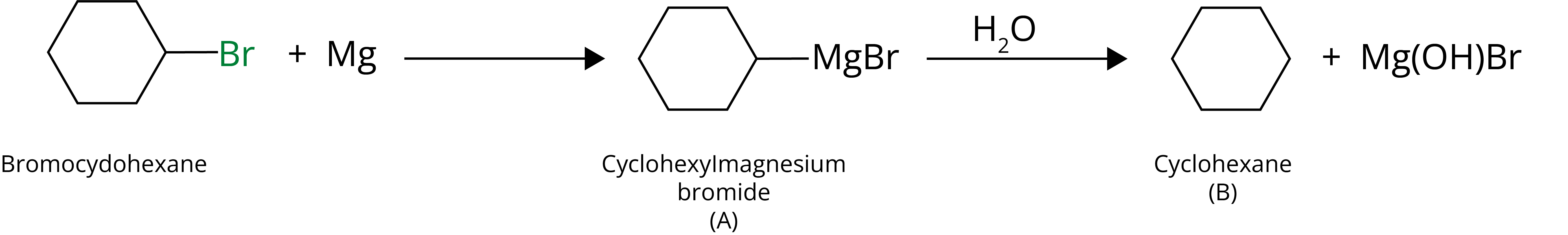
Key Points: Hydrolysis of Grignard reagent forms respective alkane.
Solved Problems of Previous Year Question From Chapter
1. The major product formed from the dehydrohalogenation of 2-bromopentane is pent2-ene. The product formed is on the basis of
Options:
1. Hund’s Rule
2. Hofmann rule
3. Huckel’s rule
4. Saytzeff’s Rule
Ans: The correct answer for this question is the fourth option.
CH3-CH2-CH(Br)-CH3 + OH- →CH3-CH2-CH=CH-CH3 + CH3-CH2-CH2-CH=CH2
81% Pent2-ene will be formed and 19% Pent1-ene will form.
Trick: According to the saytzeff’s rule the more alkylated alkene will be formed from the elimination reaction.
2. The major product of the following chemical reaction is :
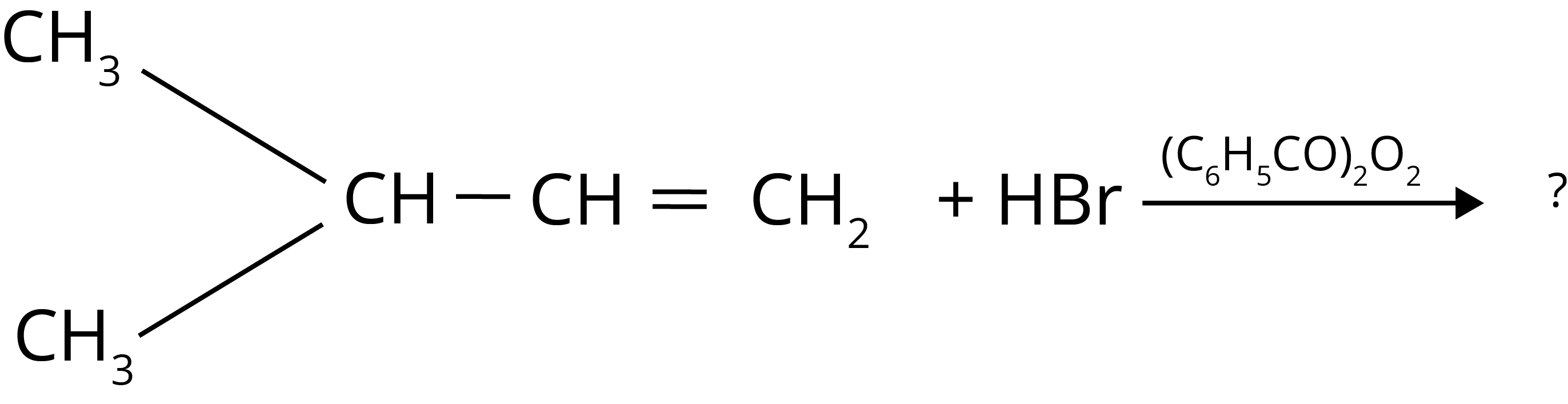
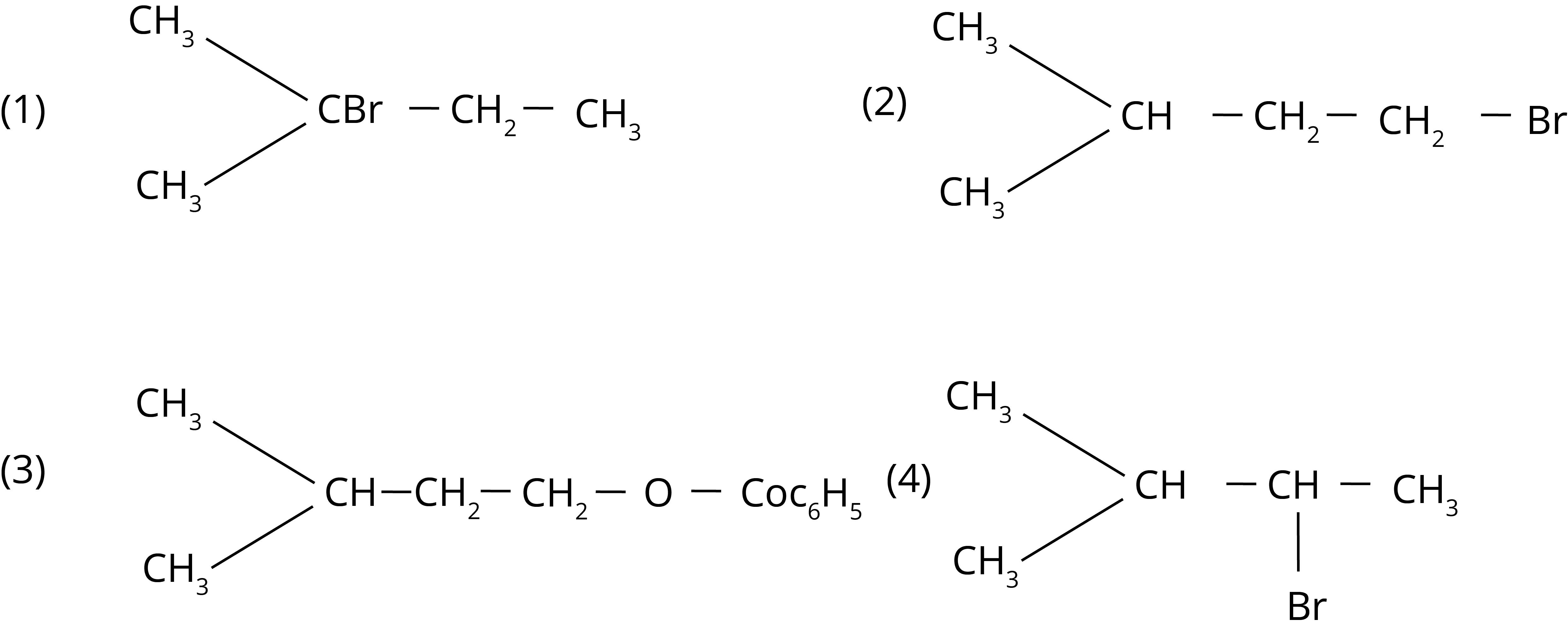
Ans: The correct answer to this question is the second option.
Trick: According to the anti-rule, Markovnikov the principal product is peroxide (based on the stability of free radical intermediate)
3. The major product formed in dehydrohalogenation reactions of 2-Bromo pentane is Pent-2-ene.
This product formation is based on?
(1) Huckel's Rule
(2) Saytzeff's Rule
(3) Hund's Rule
(4) Hofmann Rule
Ans: The correct answer is for this question is the second option.
Trick: According to the saytzeff’s rule the more alkylated alkene will be formed from the elimination reaction.
Practice Questions:
1. What will be the product of the given reaction:
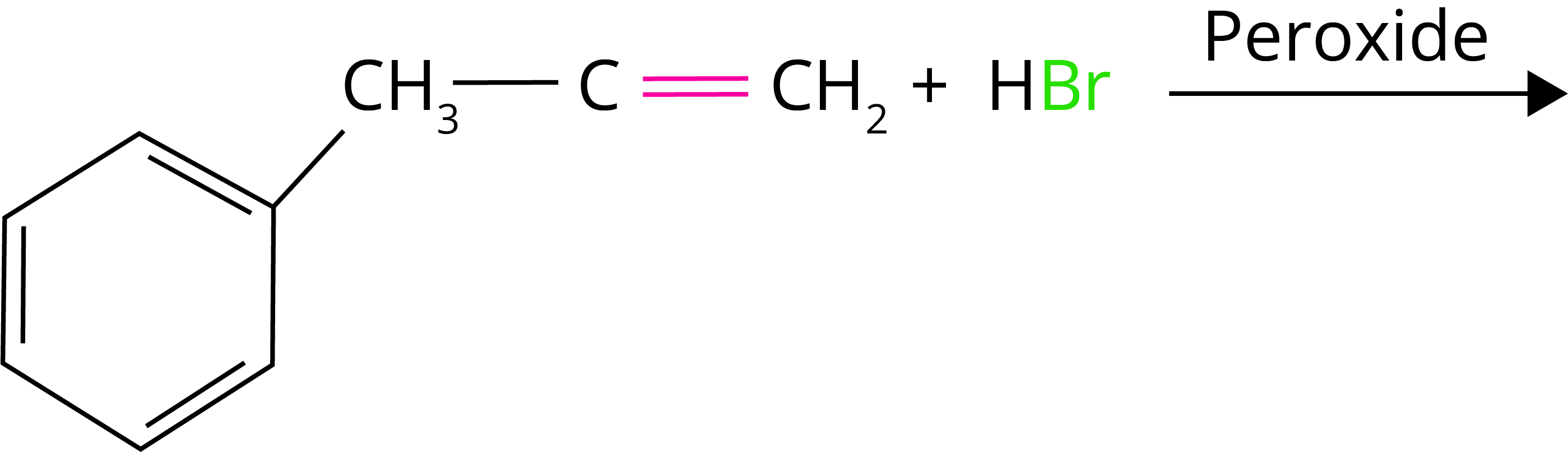
(Ans: )
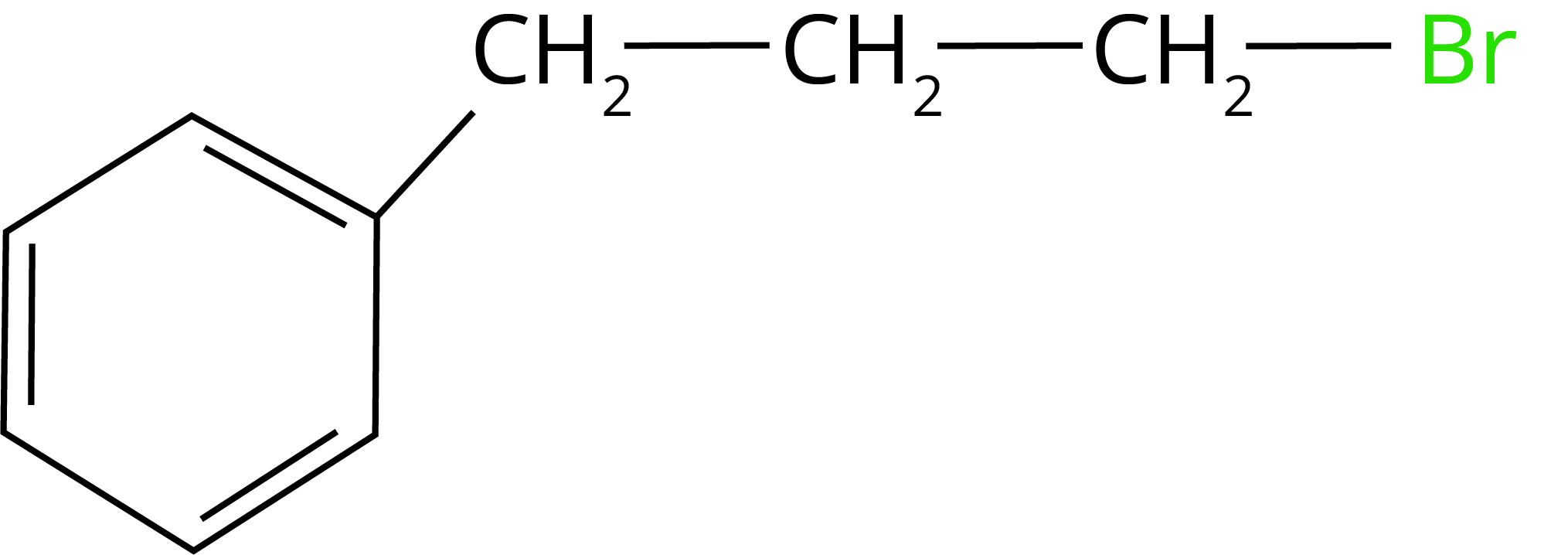
2. What happens when n-butyl chloride is treated with alcoholic KOH,
(Ans: butene will be formed)
Conclusion
In this haloalkanes and haloarenes neet note, we have provided important information regarding the chapter haloalkanes such as definition, nomenclature, important concepts, reactions, etc.. Students should work on more solved examples and previous year's question papers for securing good grades in the NEET exams.
NEET Chapter- Haloalkane and Haloarenes

 Share
ShareFAQs on NEET Chapter- Haloalkane and Haloarenes
1. What is the reaction of Frankland?
The Frankland reaction is used to make dialkyl zinc (R2Zn) from zinc (Zn) and alkyl iodide (R-I).
2. What exactly is the E2 reaction?
The E2 process involves a one-step procedure in which carbon-hydrogen and carbon-halogen bonds break to generate a double bond (C=C Pi bond).
3. Are prior year question papers sufficient for a high NEET score?
For scoring well in the NEET test, NCERT and previous year's NEET papers are sufficient. Solving previous 10-year NEET examinations gives us a significant edge because 3 to 4 questions with the same answers are sure to be repeated every year.




















 Watch Video
Watch Video